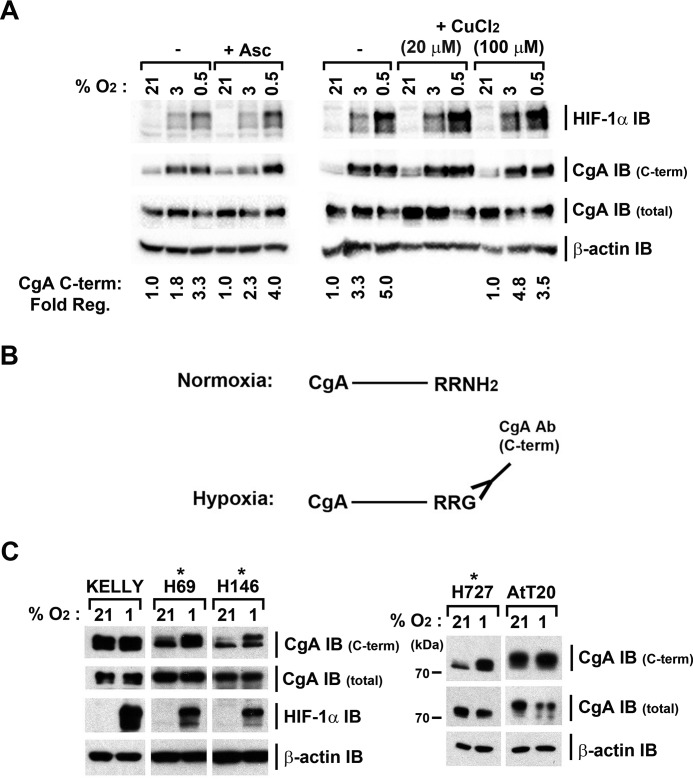
FIGURE 6.
PAM-dependent amidation of CgA is sensitive to oxygen in multiple cell lines and is not a consequence of cellular ascorbate or copper deficiency. A, supplementation of culture medium with ascorbate or copper does not alter the sensitivity of PAM to oxygen concentration. H727 control, ascorbate- or copper-supplemented cells were exposed at the indicated oxygen concentrations for 4 h. Cell extracts were IB with the indicated antibodies and PAM activity assessed by changes in CgA C-terminal immunoactivity normalized to CgA total signal. Quantitation of the fold change (at 3% and 0.5% O2) relative to 21% O2 is shown for both control and ascorbate or copper supplemented samples. The addition of ascorbate did not alter the response of PAM to oxygen concentration, but did reduce the very low level of HIF-1α detected at 21% O2, in keeping with published observations (40). B, schematic representation of the PAM and oxygen-dependent amidation at the C terminus of CgA and its consequence for interaction with the CgA C-terminal antibody. In normoxia, amidation (or amidation followed by cleavage of C-terminal CgA residues) prevents recognition by the CgA C-terminal antibody. C, IB of extracts prepared from normoxic and hypoxic (4 h at 1% O2) cells indicates that oxygen-dependent processing of the C terminus of CgA occurs in multiple independent human lung neuroendocrine cell lines. Note that the CgA in the mouse AtT20 cell line has a higher molecular weight than that detected in H727 cells.