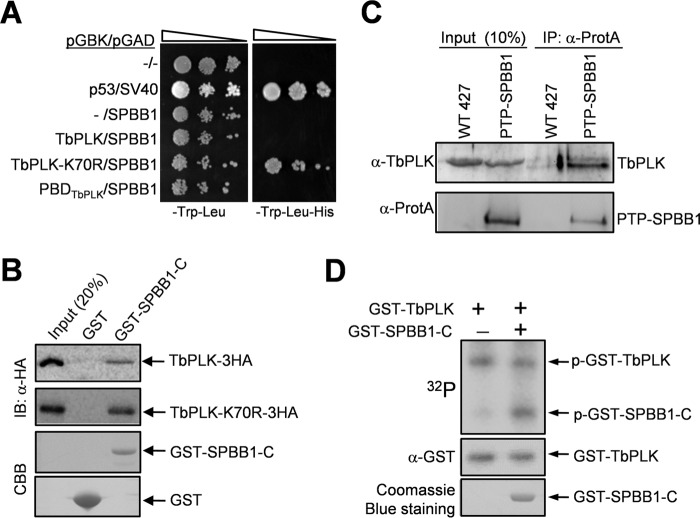
FIGURE 1.
SPBB1 interacts with TbPLK in vivo and is phosphorylated by TbPLK in vitro. A, yeast two-hybrid assays to detect the interaction between SPBB1 and TbPLK. p53/SV40 served as the positive control. −, empty vector. B, GST pulldown to detect the in vitro interaction between the C-terminal fragment of SPBB1 (SPBB1-C) and TbPLK. GST was included as the negative control. IB, immunoblot; CBB, Coomassie Brilliant Blue. C, co-immunoprecipitation (IP) of SPBB1 and TbPLK from trypanosome cell lysate. D, in vitro kinase assay to investigate the phosphorylation of SPBB1 by TbPLK. A C-terminal truncation of SPBB1 was purified as GST fusion protein for kinase assay with purified GST-fused TbPLK. GST-TbPLK was detected by Western blotting with anti-GST antibody, whereas GST-SPBB1-C was detected by Coomassie Blue staining. p-GST-TbPLK, phospho-GST-TbPLK; p-GST-SPBB1-C, phospho-GST-SPBB1-C.