FIGURE 1.
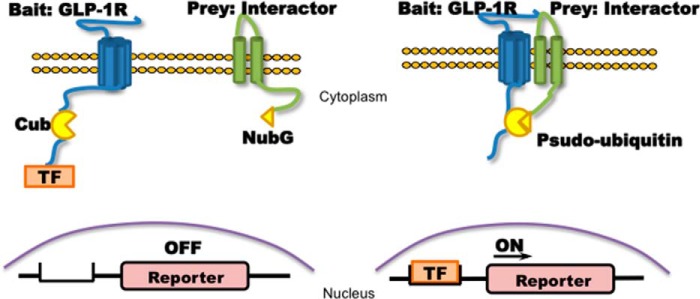
Membrane-based split ubiquitin yeast two-hybrid. Left panel, membrane-bound ubiquitin protein was split into two halves: C terminus (Cub) and N terminus (Nub, NubG is point mutation of Nub to avoid self-activation). Cub is associated with a TF that was fused to the bait GLP1R as GLP1R-Cub, and NubG was fused to the prey interactors as interactor-NubG. Right panel, if the bait interacts with the prey, the resulting proximity of the ubiquitin halves induced by the interaction will enable the reconstitution of Cub and NubG to form a functional pseudoubiquitin protein. Reconstitution recruits ubiquitin-specific proteases that cleave TF downstream of Cub, allowing the TF to translocate into the nucleus to initiate the transcription of reporter genes, which serve as readout of MYTH. As a result, the MYTH system does not rely on protein expression within the nucleus as does the traditional yeast two-hybrid system and can be used to study membrane-bound proteins such as GLP1R and its interactors.
