FIGURE 3.
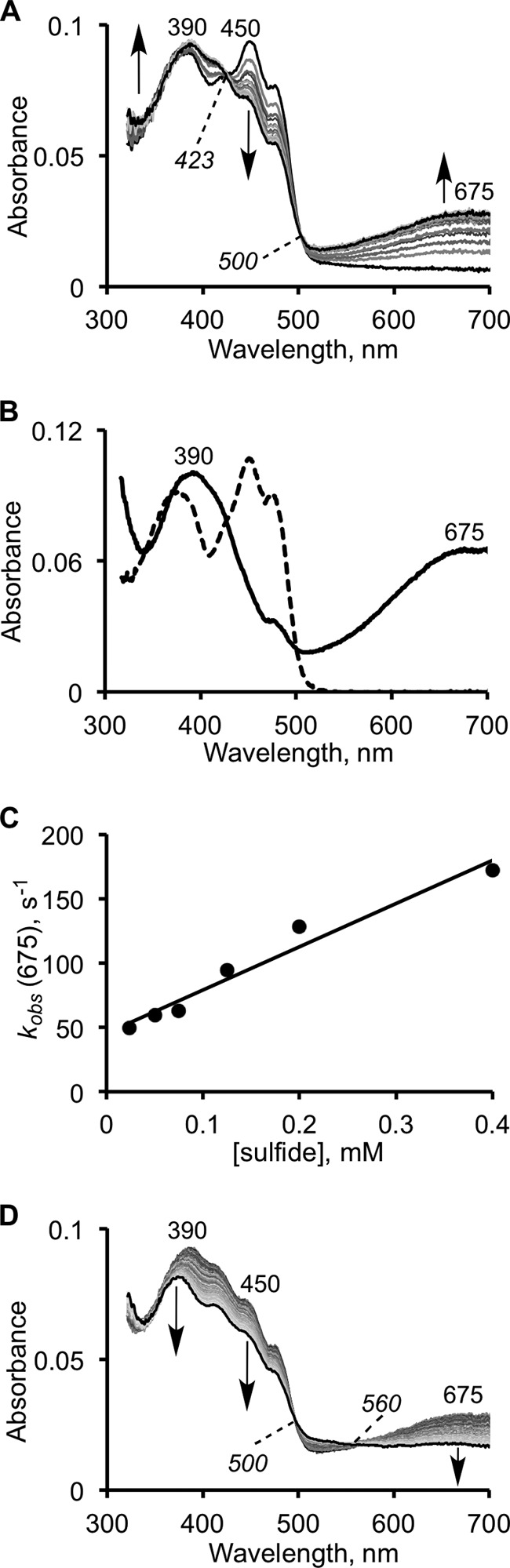
Kinetics of CT complex formation on SQR in the presence of sulfide. A, reaction between 10 μm SQR and 125 μm Na2S in 50 mm Tris-Cl buffer, pH 8.0, with 0.03% DHPC, was monitored over a period of 15 s. The first phase of the reaction is associated with the formation of an SQR-sulfide CT complex. B, representative spectrum of the fully developed CT complex (solid line) in the presence of an excess of sulfide (1 mm) and oxidized SQR (dashed line). C, dependence of the observed rate constant for formation of the CT complex on sulfide concentration, in 50 mm potassium phosphate buffer, pH 7.4, with 0.03% DHPC. The kinetics of CT complex decay (0.2 s−1) are unaffected by the changes in sulfide concentration. D, decay of the CT complex is accompanied by flavin reduction at 450 nm.
