FIGURE 2.
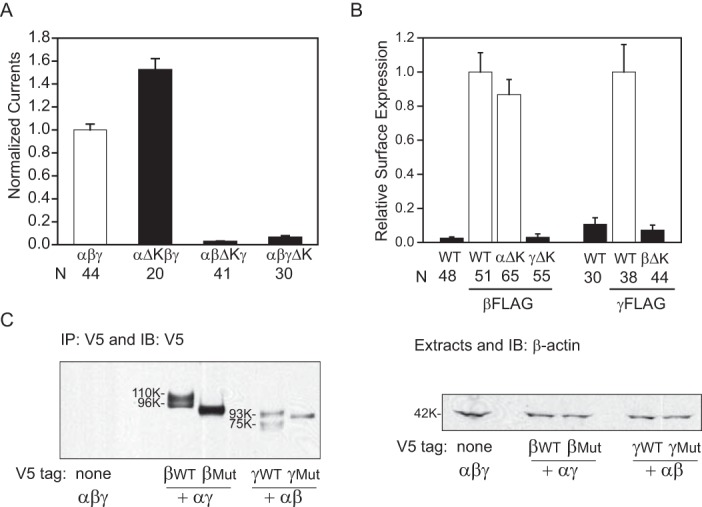
Knuckle domain deletions result in subunit-specific changes in ENaC activity and surface expression. A, normalized currents in oocytes expressing mouse αβγ, αΔKβγ, αβΔKγ, or αβγΔK ENaCs. Normalized currents represent amiloride-sensitive currents measured at −100 mV from individual oocytes, divided by the average of the amiloride-sensitive current of oocytes expressing WT channels from the same batch of oocytes. Data were pooled from two batches of oocytes. The amiloride-sensitive currents from the two batches of oocytes expressing WT ENaC were −7.5 ± 0.6 μA (n = 27) and −12.7 ± 0.7 μA (n = 17). Black columns indicate values that were significantly different from WT controls (p < 0.01). B, relative levels of surface expression. Oocytes were injected with three ENaC subunit cRNAs for αβγ (WT), αΔKβγ (αΔK), αβΔKγ (βΔK), and αβγΔK (γΔK). For α and γ mutants, a β subunit with a FLAG epitope was used, whereas a γ subunit with a FLAG epitope was used with the β subunit mutant. Relative light units measured from individual oocytes were normalized to the average relative light units of the same batch of oocytes expressing the FLAG-tagged WT control. Data were from four batches of oocytes. Black columns, values that were significantly different from the FLAG WT control group (p < 0.01). C, deletion of the knuckle domain affects processing of ENaC expressed in Xenopus oocytes. Oocytes (20 oocytes/group) were injected with αβγ cRNAs, where either the β or γ subunit had both an N-terminal HA and C-terminal V5 tag. Epitope-tagged subunits were either WT or had a knuckle domain deletion mutant (Mut), as indicated. αβγ lacking an epitope tag was used as a negative control. The following day, ENaC was immunoprecipitated (IP) from oocyte extracts with anti-V5 antibodies conjugated to beads and immunoblotted with anti-V5 antibodies. Molecular weights of the immature β (96K) and γ (93K) subunits and the mature β (110K) and γ (75K) subunits are indicated beside the immunoblots (IB) for WT subunits. Mobility of mutant subunits in each case was slightly faster than wild-type subunits, as expected for the knuckle deletions (∼3 kDa). No mature forms of the β and γ mutant subunits were observed, even upon longer exposures. An aliquot of the oocyte extract (5%) for each group was immunoblotted with anti-β-actin antibodies (42 kDa). Error bars, S.E.
