FIGURE 2.
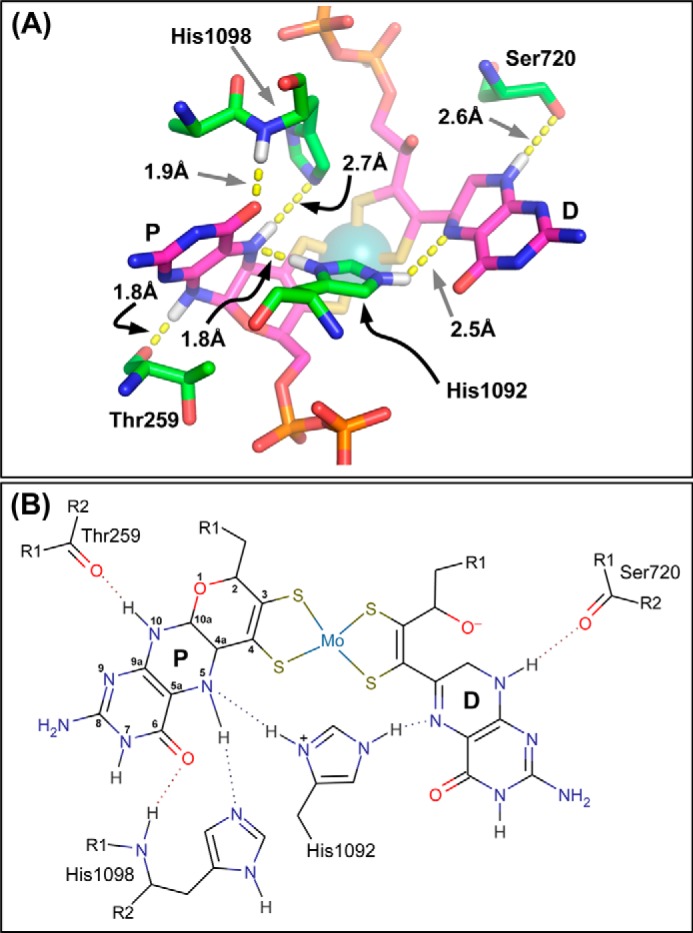
A, residues defining pyranopterin piperazine ring coordination. The image was generated using the NarGHI structure described by Protein Data Bank code 1Q16 (25). His1092 functions to bridge the two pyranopterins, with its ND1 nitrogen functioning as an H-bond donor to the proximal piperazine N-5 atom, and its NE2 nitrogen functioning as an H-bond donor to the distal piperazine N-5 atom. Note that His1092 is shown in its protonated form to facilitate H-bond donation to both piperazine N-5 atoms. For further details, see text. B, proposed H-bonding network around the piperazine rings of the two pyranopterins. The proximal pyranopterin (labeled P) is shown in its tetrahydro form, with this redox state stabilized by both the bridging His1092 and the stabilizing His1098, whereas the piperazine of the distal pyranopterin (labeled D) is shown in a form equivalent to the 10,10a-dihydro pyranopterin. In both panels, some predicted hydrogens have been added for clarity. Images were generated as described in the legend to Fig. 1.
