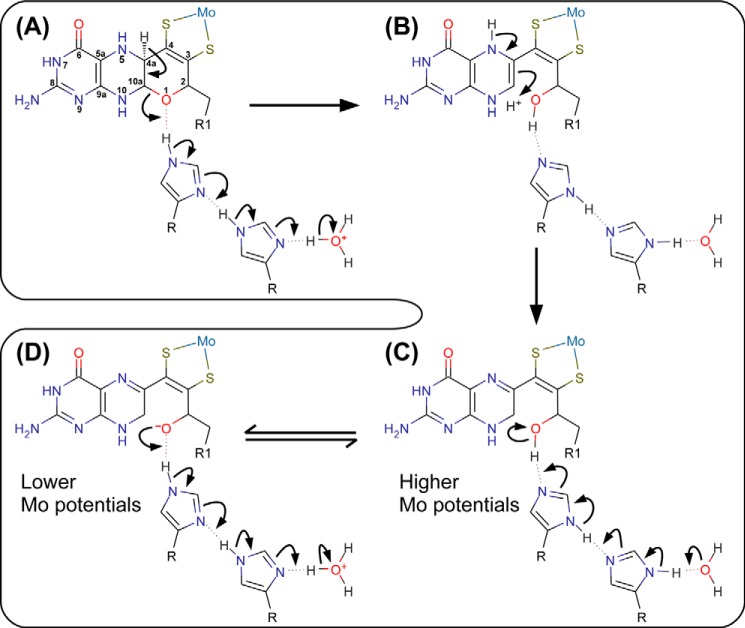
FIGURE 7.
Proposed mechanism of pyran ring opening of the distal pyranopterin of NarGHI. A, the electron transfer relay comprising NarG-His1163 and NarG-His1184 catalyzes proton abstraction from the C-4a atom and pyran ring-opening. The product of this reaction has a piperazine oxidation state and structure equivalent to the 5,10-dihydro pyranopterin (B). A tautomerization reaction results in a form equivalent to the 10,10a-dihydro pyranopterin with a protonated oxygen equivalent to O-1. The pterin core of this form is equivalent to the lowest energy dihydropterin tautomer (15, 59). C, the NarG-His1163/His1184 charge-transfer relay functions to modulate an equilibrium between the protonated (hydroxyl) and deprotonated (alkoxide) forms of the bicyclic distal pyranopterin, with the protonated form having a higher overall predicted Mo(VI/VIV) reduction potential than the deprotonated form (D).