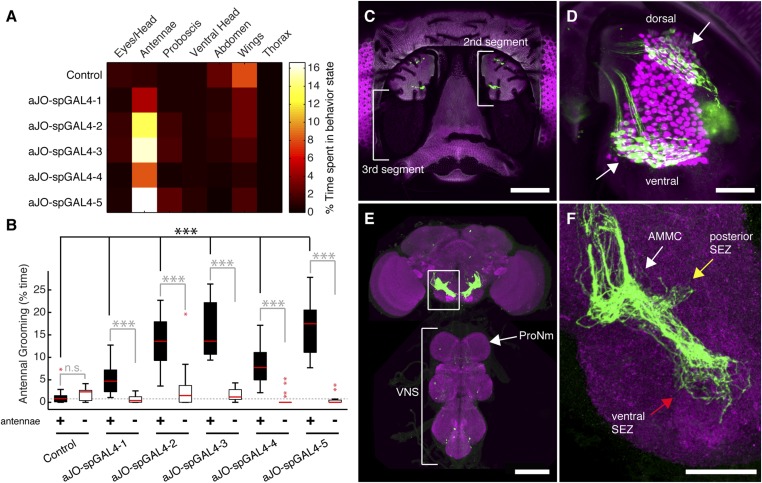
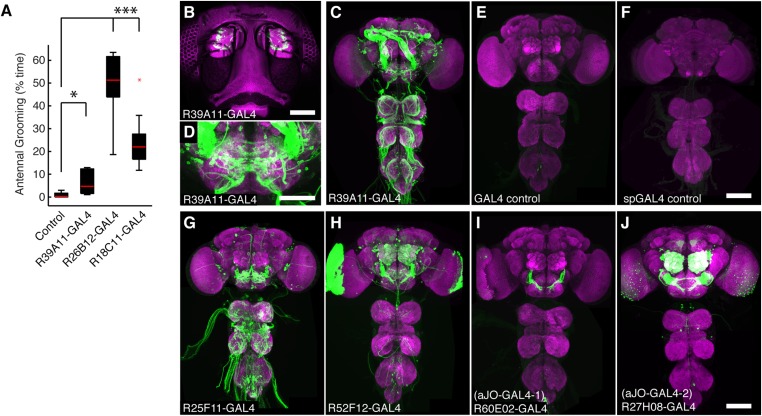
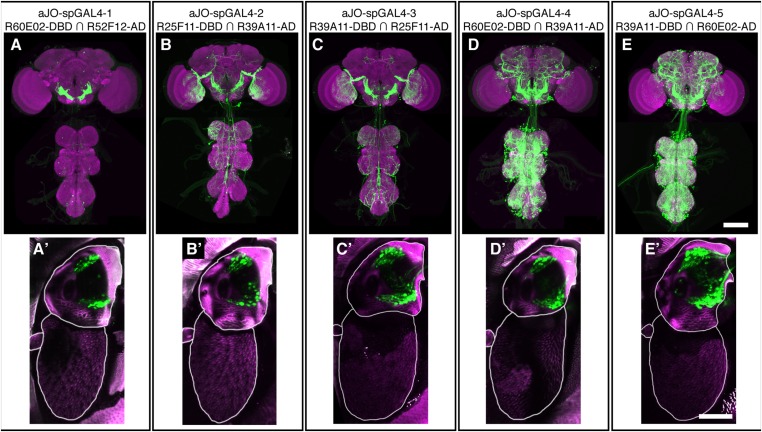
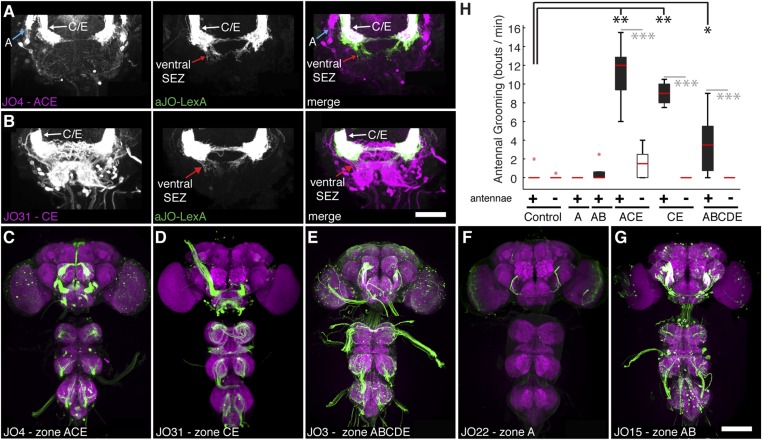
Figure 1. Sensory neurons that elicit antennal grooming.
(A) Grooming movements performed by flies in which aJO spGAL4 pairs drove expression of thermally activated dTrpA1. Movements were manually scored from 2 min of recorded video per fly (n ≥ 17 flies per spGAL4). Colors correspond to the percent of total time spent performing each movement. (B) Percent time flies spent antennal grooming with thermogenetic activation of neurons targeted by spGAL4 pairs, with or without their antennae (filled or open boxes, respectively). Bottom and top of the boxes indicate the first and third quartiles respectively; median is the red line; whiskers show the upper and lower 1.5 IQR; red dots are data outliers (n ≥ 17 for each box; asterisks show p < 0.0001, Kruskal–Wallis and post hoc Mann–Whitney U pairwise tests with Bonferroni correction). Dotted line marks the median of the intact control. (C–F) aJO-spGAL4-1 driving expression of green fluorescent protein (GFP). Maximum intensity projections are shown. (C) Frontal view of the head (native GFP fluorescence, green; cuticle autofluorescence, magenta). Left bracket shows the third antennal segment. Right bracket marks the second antennal segment, which is shown in (D). Scale bar, 100 μm. (D) Second antennal segment co-stained with anti-GFP (green) and anti-Elav (magenta, marks neuronal nuclei) antibodies. White arrows show the ventral and dorsal aJO clusters. Scale bar, 25 μm. (E, F) Central nervous system (CNS) co-stained with anti-GFP (green) and anti-Bruchpilot (magenta) to visualize the aJO afferent projections into the ventral brain neuropile (E) and their specific targeting of the indicated antennal mechanosensory and motor center (AMMC) and subesophageal zone (SEZ) regions (arrows shown in F). Box in (E) indicates region shown in F. Scale bars, (E) 100 μm and (F) 25 μm. Prothoracic neuromeres (ProNm). Ventral nervous system (VNS). See also Figure 1—figure supplement 1 and Figure 1—figure supplement 2.