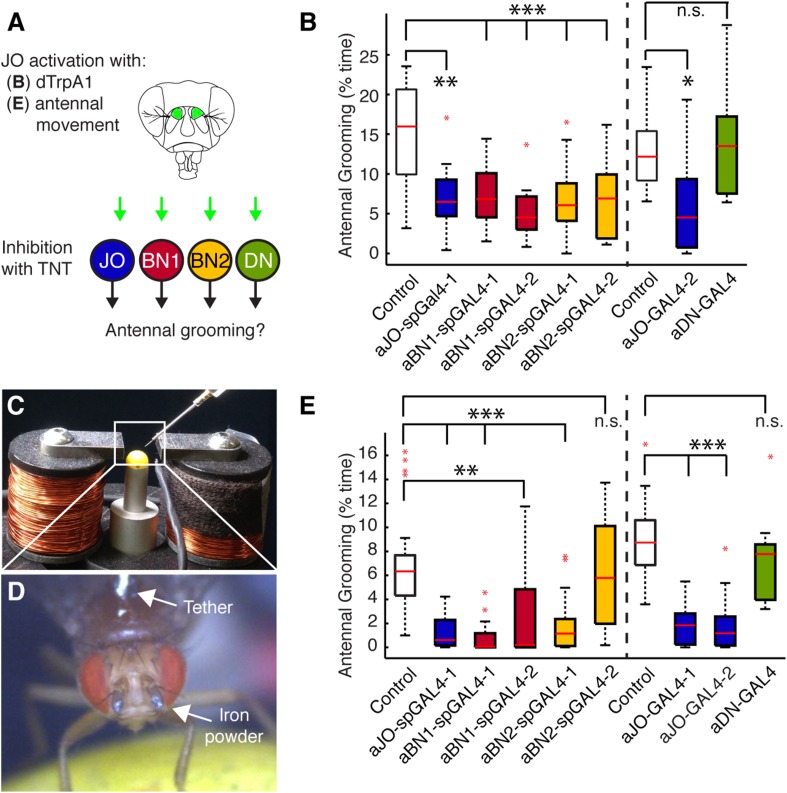
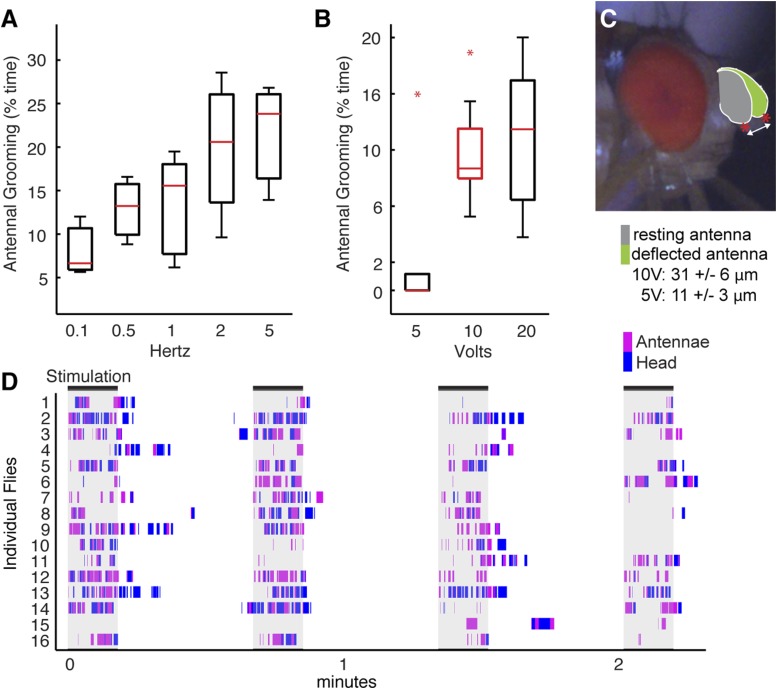
Figure 4. Functional relationships among putative antennal circuit components.
(A) Overview of experiments shown in (B, E). Grooming was induced by thermogenetic activation of Johnston's Organ (JO) neurons (dTrpA1) or by imposed displacements of the antennae. Synaptic release was blocked in different neuronal classes expressing tetanus toxin (TNT). (B) Antennal grooming performed by flies with thermogenetic activation of the aJO while inhibiting synaptic release in interneuron classes. The experiment was performed and data is displayed as described in Figure 1B (n ≥ 11 flies per spGAL4). (C–E) To displace the antennae, iron powder was glued to the third antennal segment and the flies were tethered within an electromagnet. (C) Image of the electromagnet apparatus. (D) Tethered fly with iron powder on its antennae. Magnetic pulses were delivered to displace the third antennal segment at 1 Hz for 4 × 10 s periods, with 30 s rests between stimulations. Flies were recorded and their grooming movements were manually scored (see Figure 4—figure supplement 3D for ethograms). (E) The percent time that flies spent grooming their antennae within the total assay time is shown. The grooming responses to antennal movements were also tested while blocking synaptic release in the different neuronal types with TNT. Box plots and statistics are shown as described in Figure 1B (n ≥ 11 flies per line).