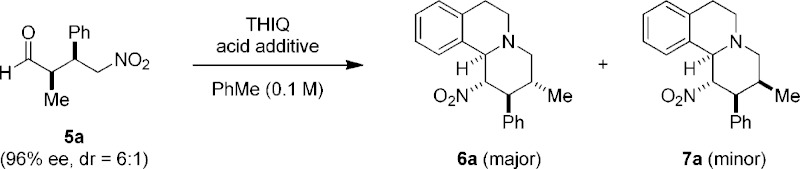
Table 1. Evaluation of Reaction Conditionsa.
| entry | acid (equiv) | temp (°C) | time (min) | yield (%) | ratio 6a:7a |
|---|---|---|---|---|---|
| 1 | 150 (μW) | 15 | complex | ND | |
| 2 | AcOH (1) | 150 (μW) | 15 | complex | ND |
| 3 | AcOH (5) | 150 (μW) | 15 | 45 | 1.8:1 |
| 4 | AcOH (10) | 150 (μW) | 15 | 65 | 2:1 |
| 5 | 2-EHA (10) | 150 (μW) | 15 | 30 | 1:1 |
| 6 | PhCO2H (10) | 150 (μW) | 15 | NRb | |
| 7c | AcOH (10) | 150 (μW) | 15 | 65 | 1.8:1 |
| 8c | AcOH (10) | 120 (μW) | 2 | 71 | 1.8:1 |
| 9c | AcOH (10) | reflux | 1 h | 71 | 2:1d |
| 10c | AcOH (10) | 60 | 15 h | 62 | 2:1 |
Reactions were performed with 5a (0.2 mmol) and THIQ (4 equiv). Product ratios were determined by 1H NMR analysis of the crude reaction mixture. Yields correspond to combined, isolated yields of both diastereomers. ND: not determined. NR: no reaction.
Partially epimerized starting material was recovered (dr = 1:1).
Performed with 5a (0.6 mmol) and 2 equiv of THIQ in the presence of 4 Å MS.
Both 6a and 7a were obtained with 95% ee.