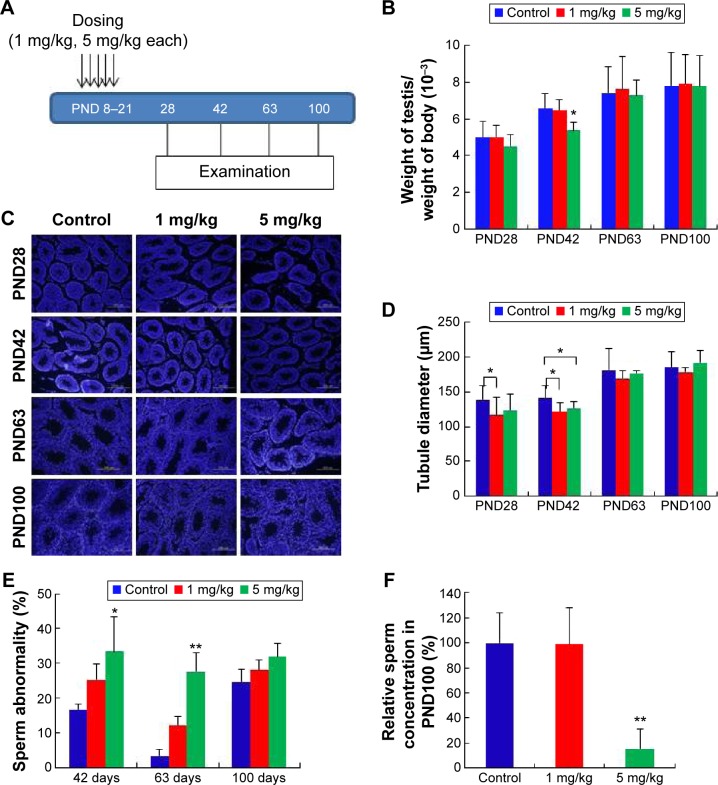
Figure 6.
Treatment of neonatal male mice with silver nanoparticles.
Notes: (A) Figure shows five doses administered over 13 days at 5 mg/kg per dose. Reproductive toxicity was assessed at postnatal day PND28, PND42, PND60, and PND100. Arrows indicate the injection of AgNPs five times from PND8 to PND21. (B) The ratio of testis weight/body weight of mice injected with silver nanoparticles or water showed no statistically significant differences, with the exception of the 5 mg/kg-treated group at PND42. (C) Histological examination of the testis using 4′,6-diamidino-2-phenylindole (DAPI). (D) The diameter of the convoluted tubules in silver nanoparticles-treated mice at PND28 and PND42 compared with the control. The diameter of the convoluted tubules in the two treatment groups shrank markedly in comparison to the control at PND42 (P<0.05). (E) Percentage of abnormal sperm from morphological examination. There was an obvious increase in the rate of abnormal sperm in the silver nanoparticles-treated groups. (F) The concentration of sperm at PND100 was significantly reduced in the 5 mg/kg-treated group (P<0.01). *P<0.05, **P<0.01.
Abbreviation: PND, postnatal day.