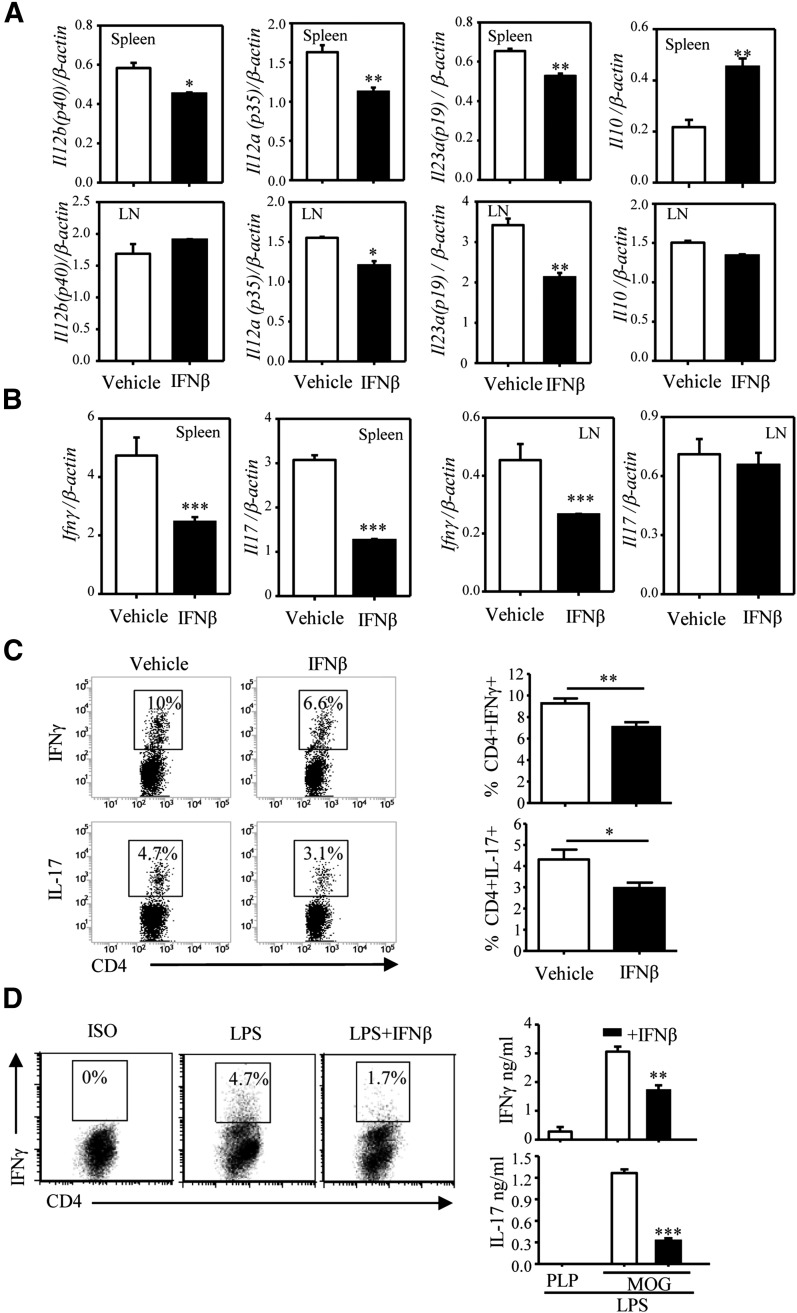
Figure 2. IFN-β affects IL-12, IL-23, and IL-10 expression and Th1/Th17 differentiation in vivo and in vitro.
C57BL/6 mice (n = 14/group) were immunized with MOG33–55 emulsified in CFA as described in the Materials and Methods section. Mice were treated with the vehicle (200 μl PBS) or IFN-β 10,000 U (in 200 μl PBS) via i.p. inoculation on days 3, 5, and 7 postimmunization. On day 8, spleens and DLNs were harvested. (A) CD11c+ cells, purified from pooled spleens and LNs (3 mice/group), were subjected to RNA extraction and qRT-PCR to measure expression of Il12a (p35), Il12b (p40), Il23a (p19), and Il10. Each sample was tested in triplicate, and the results were expressed as means ± sd. (B) RNA from pooled splenocytes and LN cells (3 mice/group) was subjected to qRT-PCR to measure expression of IFN-γ and Il17. Each sample was tested in triplicate. (C) Splenocytes (from 8 mice/group) restimulated ex vivo with PMA and ionomycin for 4 h were stained for surface CD4 and intracellular IL-17 or IFN-γ and analyzed by flow cytometry. A representative experiment is shown in the left panel. Results shown in the right panel represent the combined data from all 8 individual samples as means ± sd. *P < 0.05, **P < 0.01, ***P < 0.001, compared with the vehicle. (D) DCs were pretreated with IFN-β for 1 h, pulsed with MOG35–55, and treated with LPS (1 μg/ml) for 24 h. Following extensive washing with PBS, DCs (1 × 105 cells/ml) were cocultured with naïve 2D2 CD4+ T cells (1 × 106 cells/ml) for 3 d. Cytokine production was detected by FACS and ELISA. One of 3 independent experiments is shown. Results represent the means ± sd. **P < 0.01 and ***P < 0.001, compared with LPS alone.