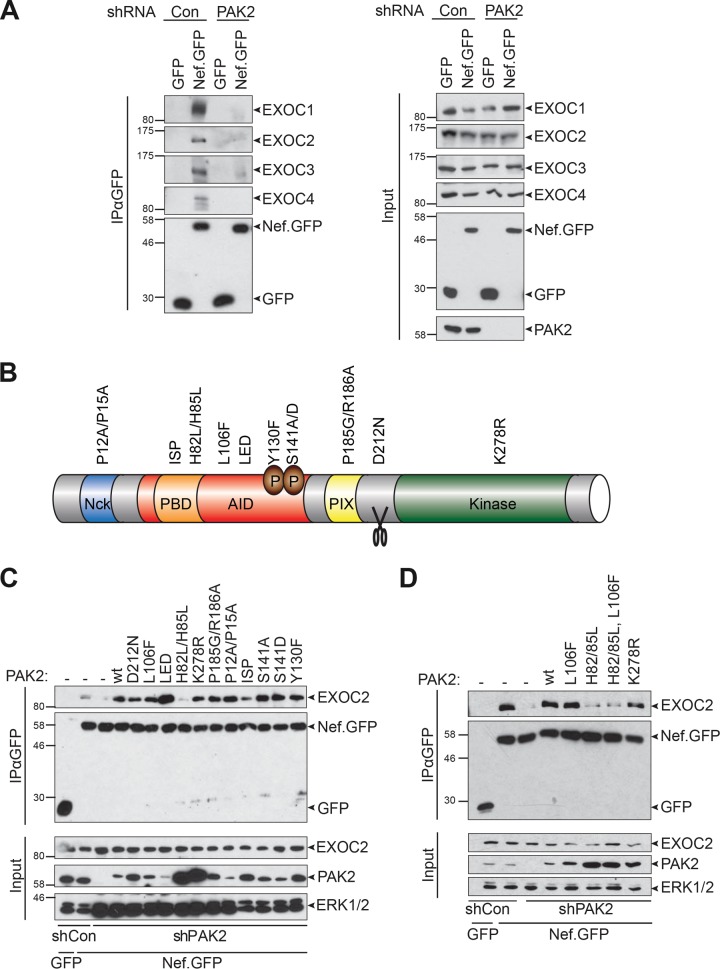
FIG 2 .
The GTPase interaction site of PAK2 is essential to facilitate interactions of Nef with EXOC. (A) Nef-EXOC coimmunoprecipitation as in Fig. 1 from Jurkat shCon and Jurkat shPAK cells transiently expressing GFP or SF2 Nef.GFP. (B) Schematic illustration of the domain organization and functionally relevant residues of PAK2. The ISP (I74N/S75P/P77A) and H82L/H85L mutant PAK2 proteins lack binding to Rac/Cdc42 because of disruption of the p21 binding domain (PBD). The L106F and K278R mutations partially disrupt PAK2 autoinhibition or abolish PAK2 activity, respectively. AID, autoinhibitory domain. (C and D) Reconstitution of Nef-EXOC coimmunoprecipitation in Jurkat shPAK2 cells by expression of shRNA-resistant PAK2 variants. Coimmunoprecipitation as in panel A but upon transient coexpression of the indicated PAK2 variants. The values to the left of the blots are molecular sizes in kilodaltons.