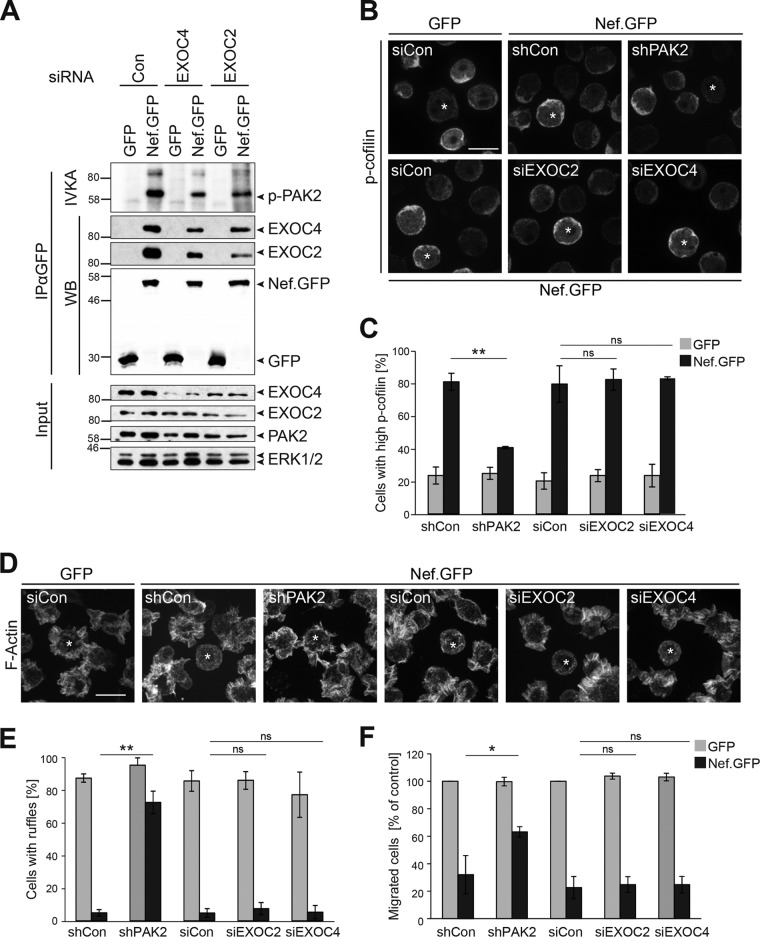
FIG 3 .
EXOC does not affect Nef-PAK2 downstream functions. (A) Nef-associated PAK2 activity. Jurkat T (TAg) lymphocytes were transfected with siRNA pools targeting EXOC2 or EXOC4 or the respective siRNA control and retransfected with a GFP or SF2 Nef.GFP expression plasmid 48 h later. At 24 h after DNA transfection, cells were lysed and subjected to GFP-Trap immunoprecipitation with subsequent IVKA. Samples were analyzed by SDS-PAGE and subjected to autoradiography and Western blotting to reveal the association of Nef with PAK2 activity (resulting in autophosphorylation of the kinase) and EXOC, respectively. The values to the left of the blots are molecular sizes in kilodaltons. (B and C) Induction of inactive p-cofilin by Nef. (B) Representative confocal micrographs of Jurkat T (CCR7) lymphocytes transiently expressing GFP or Nef.GFP in the context of shRNA-mediated depletion of PAK2, siRNA-mediated reduction of EXOC2 or EXOC4, or the respective control. Cells were plated onto coverslips, fixed, permeabilized, and stained for p-cofilin. Asterisks indicate GFP-positive cells. Scale bar, 10 µm. For dual-color images, see Fig. S4A in the supplemental material. (C) Frequency of the cells shown in panel B with high p-cofilin levels. Depicted are mean values ± standard deviations of three independent experiments with at least 100 cells counted per condition. (D and E) Inhibition of chemokine-induced actin ruffling by Nef. (D) Representative maximum projections of confocal Z-stacks of GFP- or Nef.GFP-expressing Jurkat T (CCR7) lymphocytes upon silencing of PAK2 or EXOC expression. Cells were used to seed coverslips at 24 h posttransfection with expression constructs, stimulated with 200 ng/ml SDF-1α for 20 min, fixed, permeabilized, and stained with phalloidin-TRITC to visualize F-actin. Asterisks indicate GFP-positive cells. Scale bar, 10 µm. For dual-color images, see Fig. S4B in the supplemental material. (E) Frequency of cells shown in panel D with chemokine-induced ruffles. Depicted are mean values ± standard deviations of three independent experiments with at least 100 cells counted per condition. (F) Chemotaxis toward SDF-1α. Cells shown in panel D were starved and allowed to migrate through a 5-µm porous transwell filter toward an SDF-1α gradient for 2 h. Migrated cells were quantified by flow cytometry, and data are plotted relative to the corresponding GFP control, which was set to 100%. Depicted are mean values ± standard deviations of three independent experiments each performed in triplicate. Statistical significance was assessed by Student’s t test. ns, not significant; *, P < 0.05; **, P < 0.01.