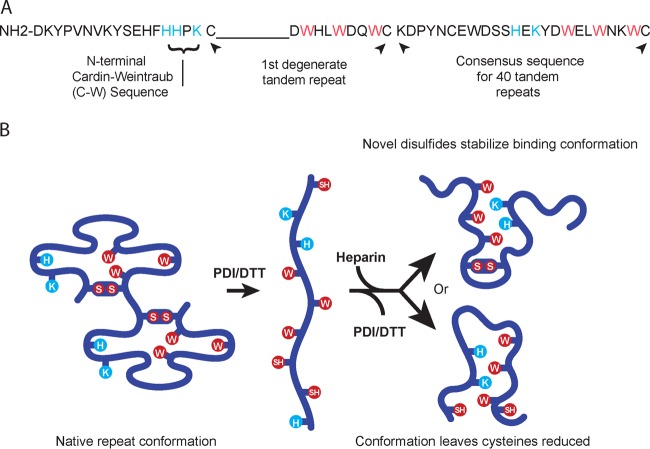
FIG 1 .
The BAD-1 adhesin. (A) The N-terminal region is only 18 amino acids long and contains a Cardin-Weintraub domain (BBxB). Immediately adjacent to this region, the first tandem repeat is degenerate, lacking a span of nine residues typical of the consensus repeats. Residues with basic (positively charged) side chains are displayed in blue, and conserved tryptophans are shown in red. The degenerate repeat is followed by the consensus sequence of the other 40 tandem repeats. (B) Repeats in the native conformation (two repeats are depicted) are predicted to hold the elements of a heparin-binding motif apart (2). Blue and red residues are as noted for panel A. Upon reduction of disulfide bonds, these elements would be able to interact freely and thus create a TSP-1-like heparin-binding cleft (2). This motif could form in one of two possible ways: with cysteines joined in novel disulfides (above) or remaining free (below).