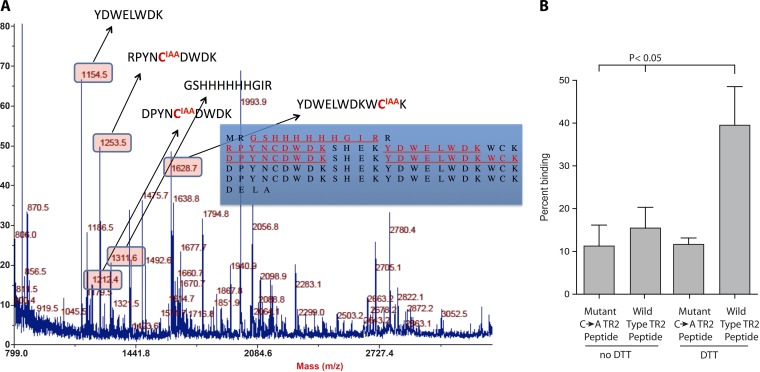
FIG 6 .
Analysis of the redox state of cysteine thiols in tandem repeat peptide models bound to heparin. (A) LC-MS/MS was used to determine whether the thiols that are reduced to permit TR4 repeats to engage heparin remain free (reduced) in the binding conformation, or if this conformation favors the formation of novel disulfide pairings. In the bound conformation, free thiols were carboxymethylated with IAA. After PAGE separation, reduction, and tryptic digestion, all other cysteines were labeled with NEM. Detected tryptic peptides are shown in red and underlined (inset). Corresponding peptide masses appear in the boxes, and modified cysteines are shown in red. (B) Peptide binding to heparin-agarose. Wild-type TR2 synthetic peptide comprises two tandem repeats flanked by two respective pairs of cysteines, while the mutant (C → A) TR2 peptide is two tandem repeats in which the cysteines have been replaced with alanines. Peptides were treated with 10 mM DTT before analysis of binding to heparin-agarose.