Table 3.
Screening of the reaction conditions using iodide salts[a]
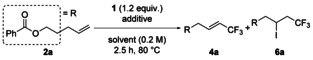 | |||||
|---|---|---|---|---|---|
| Entry | Iodide salts | Equiv | Solvent | Yield of4 a[%][b] | Yield of6 a[%][b] |
| 1 | TBAI | 1.0 | DMF | 21[c] | 0 |
| 2 | TBAI | 1.0 | MeCN | 19[d] | trace |
| 3 | TBAI | 1.0 | MeOH | 16[e] | 3 |
| 4 | TBAI | 1.0 | 1,4-dioxane | 37[f] | 0 |
| 5[g,h,i] | TBAI | 0.3 | 1,4-dioxane | 78[e,j] | 0 |
| 6 | I2 | 1.0 | 1,4-dioxane | 0 | 3 |
| 7 | NaI | 1.0 | 1,4-dioxane | trace | 50 |
| 8 | KI | 1.0 | 1,4-dioxane | trace | 61 |
| 9 | CsI | 1.0 | 1,4-dioxane | trace | 72 |
| 10 | KI | 1.0 | DMF | 22[c] | 0 |
| 11 | CsI | 1.0 | DMF | 30[k] | 0 |
| 12[g] | CsI | 1.1 | 1,4-dioxane | 0 | 88 |
| 13[g,l] | KI | 1.1 | 1,4-dioxane | 0 | 92[j] |
[a] The reactions were carried out with Togni reagent 1 (1.2 equiv) and additive on a 0.25 mmol scale, unless otherwise noted. [b] Determined by 19F NMR analysis using fluorobenzene as an internal standard. [c] The E/Z ratio was 6:1. [d] The E/Z ratio was 17:2. [e] The E/Z ratio was 7:1. [f] The E/Z ratio was 8:1. [g] Run with 1.5 equiv of Togni reagent 1. [h] Run for 12 h. [i] Run in 0.5 m solution. [j] Yield of isolated product. [k] The E/Z ratio was 13:2. [l] Run at 60 °C for 9 h.
