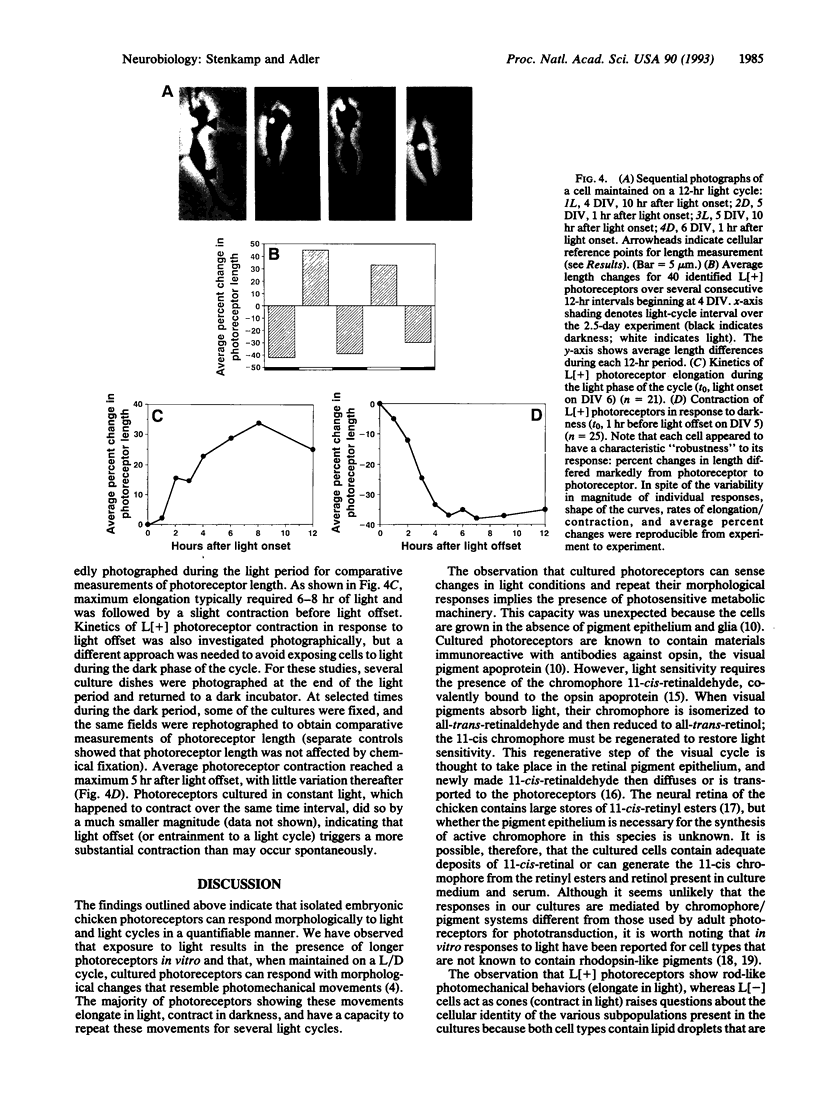
Abstract
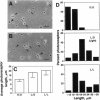
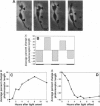
Isolated retinal precursor cells, grown without pigment epithelial or glial cells and in the absence of intercellular contacts, develop a complex set of photoreceptor-specific properties, including polarized structural and molecular organization and opsin immunoreactivity. We report here that these isolated embryonic photoreceptors are also capable of responding to light. Sequential photography showed that 50% of the photoreceptors grown in a light cycle elongate when exposed to light and contract in response to darkness. A smaller population (20%) showed the opposite response. Responses of individual cells could be observed during several sequential light cycles and resemble photomechanical movements in vivo [Ali, M. A. (1971) Vision Res. 11, 1225-1288]. The differentiation program expressed by isolated precursor cells, therefore, includes the capacity for highly complex functional activities that require light sensitivity. These observations raise challenging questions regarding the nature of the chromophore and pigments that mediate light-regulated behaviors of cultured photoreceptors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R. Developmental predetermination of the structural and molecular polarization of photoreceptor cells. Dev Biol. 1986 Oct;117(2):520–527. doi: 10.1016/0012-1606(86)90319-2. [DOI] [PubMed] [Google Scholar]
- Adler R., Hatlee M. Plasticity and differentiation of embryonic retinal cells after terminal mitosis. Science. 1989 Jan 20;243(4889):391–393. doi: 10.1126/science.2911751. [DOI] [PubMed] [Google Scholar]
- Adler R., Lindsey J. D., Elsner C. L. Expression of cone-like properties by chick embryo neural retina cells in glial-free monolayer cultures. J Cell Biol. 1984 Sep;99(3):1173–1178. doi: 10.1083/jcb.99.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht-Buehler G. Surface extensions of 3T3 cells towards distant infrared light sources. J Cell Biol. 1991 Aug;114(3):493–502. doi: 10.1083/jcb.114.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M. A. Les réponses rétinomotrices: caractères et mécanismes. Vision Res. 1971 Nov;11(11):1225–1288. doi: 10.1016/0042-6989(71)90010-1. [DOI] [PubMed] [Google Scholar]
- Bernstein P. S., Law W. C., Rando R. R. Isomerization of all-trans-retinoids to 11-cis-retinoids in vitro. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1849–1853. doi: 10.1073/pnas.84.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D. Retinal photoreceptor-pigment epithelium interactions. Friedenwald lecture. Invest Ophthalmol Vis Sci. 1985 Dec;26(12):1659–1694. [PubMed] [Google Scholar]
- Bridges C. D., Alvarez R. A., Fong S. L., Liou G. I., Ulshafer R. J. Rhodopsin, vitamin A, and interstitial retinol-binding protein in the rd chicken. Invest Ophthalmol Vis Sci. 1987 Apr;28(4):613–617. [PubMed] [Google Scholar]
- Dubocovich M. L. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983 Dec 22;306(5945):782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- Giebultowicz J. M., Riemann J. G., Raina A. K., Ridgway R. L. Circadian system controlling release of sperm in the insect testes. Science. 1989 Sep 8;245(4922):1098–1100. doi: 10.1126/science.245.4922.1098. [DOI] [PubMed] [Google Scholar]
- Iuvone P. M., Avendano G., Butler B. J., Adler R. Cyclic AMP-dependent induction of serotonin N-acetyltransferase activity in photoreceptor-enriched chick retinal cell cultures: characterization and inhibition by dopamine. J Neurochem. 1990 Aug;55(2):673–682. doi: 10.1111/j.1471-4159.1990.tb04186.x. [DOI] [PubMed] [Google Scholar]
- Iuvone P. M. Development of melatonin synthesis in chicken retina: regulation of serotonin N-acetyltransferase activity by light, circadian oscillators, and cyclic AMP. J Neurochem. 1990 May;54(5):1562–1568. doi: 10.1111/j.1471-4159.1990.tb01205.x. [DOI] [PubMed] [Google Scholar]
- Iuvone P. M. Regulation of retinal dopamine biosynthesis and tyrosine hydroxylase activity by light. Fed Proc. 1984 Sep;43(12):2709–2713. [PubMed] [Google Scholar]
- Jones G. J., Crouch R. K., Wiggert B., Cornwall M. C., Chader G. J. Retinoid requirements for recovery of sensitivity after visual-pigment bleaching in isolated photoreceptors. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9606–9610. doi: 10.1073/pnas.86.23.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot J. I., Fernald R. D. Circadian rhythm and light regulate opsin mRNA in rod photoreceptors. Nature. 1989 Feb 2;337(6206):454–457. doi: 10.1038/337454a0. [DOI] [PubMed] [Google Scholar]
- Kramer S. G. Dopamine: A retinal neurotransmitter. I. Retinal uptake, storage, and light-stimulated release of H3-dopamine in vivo. Invest Ophthalmol. 1971 Jun;10(6):438–452. [PubMed] [Google Scholar]
- Madreperla S. A., Adler R. Opposing microtubule- and actin-dependent forces in the development and maintenance of structural polarity in retinal photoreceptors. Dev Biol. 1989 Jan;131(1):149–160. doi: 10.1016/s0012-1606(89)80046-6. [DOI] [PubMed] [Google Scholar]
- Politi L. E., Adler R. Generation of enriched populations of cultured photoreceptor cells. Invest Ophthalmol Vis Sci. 1986 May;27(5):656–665. [PubMed] [Google Scholar]
- Uehara F., Matthes M. T., Yasumura D., LaVail M. M. Light-evoked changes in the interphotoreceptor matrix. Science. 1990 Jun 29;248(4963):1633–1636. doi: 10.1126/science.2194288. [DOI] [PubMed] [Google Scholar]
- Wang S. Z., Adler R., Nathans J. A visual pigment from chicken that resembles rhodopsin: amino acid sequence, gene structure, and functional expression. Biochemistry. 1992 Apr 7;31(13):3309–3315. doi: 10.1021/bi00128a002. [DOI] [PubMed] [Google Scholar]