Abstract
Hepatitis C virus (HCV) genotypes’ monitoring allows real-time insight into the dynamic changes that occur in the global epidemiological picture of HCV infection. Intravenous drug use is currently the primary driver for HCV transmission in developed and developing countries. The distribution of HCV genotypes/subtypes differs significantly between people who inject drugs (PWID) and the general population. HCV genotypes that previously exhibited a limited geographical distribution (3a, 4) are becoming more prevalent in this high-risk group. Immigration from HCV-endemic countries and the evolving networks of HCV transmission in PWID influence HCV genotypes distribution in Europe. Social vulnerabilities (e.g., unemployment, homelessness, and limited access to social and healthcare insurances systems) are important triggers for illicit drug use, which increases the associated risks of HCV infection and the frequent emergence of less prevalent genotypes. Genotype/subtype determination bears important clinical consequences in the progression of liver disease, susceptibility to antiviral therapies and the emergence of resistance-associated variants. An estimated half of the chronically HCV-infected PWID are unaware of their infection, and only one in ten of those diagnosed enter treatment. Nevertheless, PWID exhibit high response rates to new antiviral regimens, and the level of HCV reinfection is unexpectedly low. The focus of the healthcare system must be on the early detection and treatment of infection, to avoid late presentations that are associated with high levels of viremia and liver fibrosis, which may diminish the therapeutic success rate.
Keywords: Hepatitis C, Hepatitis C virus genotypes, Intravenous drug use, People who inject drugs, Direct-acting antivirals
Core tip: Careful surveillance of circulating hepatitis C virus (HCV) genotypes/subtypes is compulsory to reconstruct the natural history of HCV epidemics and viral transmission chains in high-risk populations, such as people who inject drugs (PWIDs). Genotypes 1a and 3a predominate among PWID worldwide, but genotype 4 has been reported with increased frequency. This review analyzes the factors that underlie the different distributions of HCV genotypes in PWID relative to the general population and highlights the need for early diagnosis and care in this vulnerable group, which responds well to new antiviral therapies and exhibits unexpectedly low reinfection rates.
INTRODUCTION
Non-communicable diseases have replaced infectious diseases as the most important causes of morbidity in the general population in the last two decades. Communicable diseases accounted for 24.9% of the total 52.8 million deaths reported worldwide in 2010, which is an important decrease relative to 1990, when these diseases were responsible for 34.1% of 46.5 million deaths[1]. Human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS), tuberculosis and chronic viral hepatitis are important exceptions. There are significant regional variations in this trend[2], which highlight the importance of continuous epidemiological monitoring of all diseases with public health relevance. Chronic hepatitis C is a significant cause of liver-related morbidity and mortality. There are more than 180 million persons chronically infected with the hepatitis C virus (HCV) worldwide who are at risk of developing liver cirrhosis, end-stage liver disease and hepatocellular carcinoma. An additional 3-4 million persons are newly infected each year[3]. It is estimated that 57% and 78% of patients with active viral replication will develop cirrhosis and hepatocellular carcinoma, respectively, within two or three decades in the absence of antiviral treatment, with 500000 deaths reported annually[4]. A large community-based Australian study demonstrated that people with hepatitis C exhibited a significantly increased risk of liver-related deaths compared with the general population[5].
HCV belongs to the Flaviviridae family, Hepacivirus genus. Humans are the only reservoir for HCV, but experimental infection in chimpanzees is possible. New members of the Hepacivirus and the related Pegivirus genera (pathogens for dogs and horses) have been recently discovered in rodents and bats, which serve as models for HCV biological studies[6,7]. HCV is an enveloped, positive single-stranded RNA virus, and its genome encodes three structural (core and envelope E1 and E2) and seven non-structural (NS) genes. Three NS genes are essential for the viral replicative cycle, and these genes are targets for direct-acting antivirals (DAA)[8]: (1) NS3-4A protease, which is involved in post-translational viral protein processing; (2) NS5B viral polymerase, which directs nucleic acid replication; and (3) NS5A, which encodes a phosphoprotein that participates in genome replication and the assembly of progeny virions.
The error-prone nature of the HCV NS5B polymerase and the accumulation of mutations in a small hypervariable region in the envelope-encoding genes generate a high level of variability. This variability is translated in the existence of 7 major HCV genotypes (with 30%-35% variation at the nucleotide level); 67 subtypes (with less than 15% difference at the nucleotides level), each composed by a myriad of viral quasispecies; and 9 recombinant forms (e.g., the most frequently reported, G2k/1b, which is represented by multiple isolates)[9,10]. Each genotype exhibits a different degree of variability: 7 subtypes in G1; 11 subtypes in G2; 6 subtypes in G3; 17 subtypes in G4; 24 subtypes in G6; and only 1 subtype in G5 and 7. There are multiple consequences related to this enormous viral heterogeneity: (1) reinfections with a different genotype are possible because of the very limited cross-antigenicity; (2) the emergence of immune-escape mutants, which accounts for the high rate of chronic infections; (3) the therapeutic response is genotype- and subtype-specific; and (4) the selection of viral-resistant strains contribute to the need for combination therapies.
The most important method of HCV spreading is parenteral transmission via intravenous drug use, unsafe medical procedures, including breaches in injection safety and infection prevention practices in hospitals, and the administration of unscreened blood products[11,12]. Approximately 80% of all HCV cases are concentrated in low- and middle-income countries in the Middle East, North Africa, South and East Asia (Table 1). The prevalence of HCV in North America is generally low (< 1.5%), with an increase to 5.4%-20% in military veterans[13]. The estimated mean prevalence of HCV infection is 1.03% in Europe, but large geographical variations are registered, from less than 0.2% in the Northern countries to approximately 1% in the Western countries. The highest rates are reported in Romania (3.3%) and rural areas in Greece and Italy[14,15]. The most affected age group is 25-34 years, the notification rates are 22.3 in men vs 13.3 in women per 100000 population. However, the male-to-female ratio varies considerably between countries and ranges from 0.6 in Romania to 17.7 in the Netherlands[15,16].
Table 1.
The burden of hepatitis C virus infection in the WHO regions and the proportion of people who inject drugs
| WHO regions | Population (millions)1 | Estimated HCV seroprevalence2 (%) | Estimated prevalence of viremic persons3 (%) | Proportion of PWID lowest-highest estimates4 |
| Africa | 1396 | 1.0-5.3 | 0.6-4.1 | 55-97 |
| Latin America | 572 | 0.9-1.3 | 0.6-1.0 | 69-96 |
| North America | 355 | 1.3 | 0.8-1.0 | |
| Europe | 751 | 0.9-3.3 | 0.6-2.3 | 36-69 |
| Asia | 3985 | 1.1-5.4 | 0.7-2.3 | 50-53 |
Data sources:
World health statistics, 2014 (available from: www.who.int/world_health_statistics);
Mohd Hanafiah K, 2013[3];
Gower E, 2014[10] and Global Health Observatory Data Repository (http://apps.who.int/gho/data);
World Drug report 2014[12]. PWID: Proportion of people who inject drugs; HCV: Hepatitis C virus.
The seroprevalence data must be interpreted cautiously, because the methodology for HCV screening is not uniform across different regions. National estimates in Europe sometimes derive from targeted studies in specific regions of a single country, in non-clinical settings, or in selected populations[17]. A targeted screening strategy involves the testing of persons who are at risk for acquiring HCV infection (e.g., drug users, HIV-infected subjects, inmates, migrants from endemic countries, etc.) or persons with clinical signs or biochemical modifications that are suggestive of liver disease. The Center for Diseases Control (CDC) in the United States recommended HCV birth-cohort screening for persons born from 1945 to 1965[18,19]. A strategy aimed at reducing the discrepancies in reporting and promoting early HCV diagnosis and access to treatment is needed in Europe. Persons who exhibit positive HCV antibody results must be tested for active viral replication to confirm the diagnosis and assess the need for HCV therapy[20-22].
INTRAVENOUS DRUG USE - THE PRIMARY DRIVER OF HCV TRANSMISSION
People who inject drugs (PWID) account for 0.2%-0.5% of the world’s population, but represent approximately 6.8% of persons infected with HCV[12,23]. The global seroprevalence of HCV infection in PWID is approximately 51%, which means that at least 7.2 million PWID are living with HCV[12].
It is estimated that 1980000 years of life were lost because of drug dependence in 2010, and 494000 years of life were lost because of HCV infection associated with unsafe intravenous drug use (IDU)[23].
China, Russia, the United States and Brazil are home to the largest drug-injecting populations[24], with an estimated 1.3-1.6 million PWID infected with HCV per country[25]. High HCV seroprevalence rates that reach 80% are also reported in PWID from Mexico, Pakistan and Thailand[24,25]. Almost half of the 590000 people aged 18-29 years who reported intravenous drug use in the US are HCV infected[26,27], and the seroprevalence rates reach 98.7% in people who have used drugs for more than 30 years[28,29].
IDU is the most commonly reported HCV transmission route in Europe, and it represents the main risk factor for acute (33.3%) and chronic hepatitis C cases (83.7%)[14,30]. IDU is becoming prevalent in Northern and Southern European countries, where it is replacing the iatrogenic transmission that was recorded for decades[31,32]. Almost all European countries exhibit high HCV seroprevalence rates in PWID, with only the Czech Republic, Hungary and Slovenia reporting levels under 30%[33]. Table 2 presents a more detailed picture of the current levels of HCV infection in the top ten most populated European countries that are representative of this geographical region.
Table 2.
Hepatitis C virus infection prevalence in the general population and in people who inject drugs in the most populated European countries
| Country | Population (millions)1 | Injecting drug use (rate/1000 inhabitants)2 | HCV prevalence in the general population3 | HCV prevalence in PWID4 |
| Germany | 80.4 | 4.25-5.04 | 0.7 | 51 |
| France | 65.6 | 6.7-8.8 | 0.85 | 73 |
| United Kingdom | 63.7 | 3.3 | 0.6 | 47.9 |
| Italy | 59.5 | 10 | 3.0 | 61.0-64.8 |
| Spain | 41.0 | 0.2 | 1.5 | 73.3-85.9 |
| Poland | 38.5 | 2.9 | 1.5 | 44.3 72.4 |
| Romania | 20.0 | NA | 2.1-2.4 | 82.4 |
| Netherlands | 16.7 | 0.2 | 0.2 | 50-86 |
| Greece | 10.9 | 1.1 | > 1.5 | 60-73 |
| Sweden | 9.5 | 4.9 | 0.5 | 83 |
Data sources:
World health statistics 2014 (available from: www.who.int/world_health_statistics; 2,4ECMDDA, 2013[33];
An alarming rising trend in HCV seroprevalence in PWID was observed in several European countries in 2005, including Austria, Bulgaria, Cyprus, Greece and Romania. Very high levels in the incidence and prevalence of drug-associated HCV infection were also reported in 2013 in Latvia, Portugal, Turkey and Cyprus[33,34]. In contrast, the figures for Germany, France, the United Kingdom and Italy exhibited a downward trend from previous years[31,33], which reflects good performances in case-finding and case-screening approaches.
An upsurge in the prevalence of HCV infection is an epidemiological indicator of injection-related HIV infection risk in PWID[35,36]. For example, high rates of HCV infection in PWID preceded by several years important HIV outbreaks in Greece and Romania[37]. A recent meta-analysis demonstrated that the incidence of HCV infection in PWID in the European Union (EU) was as high as 66/100 person-years, and half of the chronically infected PWID were unaware of their infection status[34].
Significant risk factors for drug-associated infectious diseases have been identified in many European countries[33,38-42]: (1) a switch to drugs that allow a higher injection frequency, such as new psychotropic substances; (2) decreases in needle and syringe coverage (< 100 syringes per PWID per year, which represents a low coverage level even for HIV transmission) were reported in Romania, Greece, Cyprus, Slovakia, Hungary, Belgium and Norway; and (3) low levels (< 30%) of substitution treatment coverage, reported in Cyprus, Latvia, Lithuania, Hungary, Poland and Slovakia.
These data highlight the continuous potential for HCV-HIV epidemics to spread throughout Europe and jeopardize the efforts to decrease or stabilize the seroprevalence of blood-borne infections.
THE DISTRIBUTION OF HCV GENOTYPES IN THE GENERAL POPULATION
HCV genotypes and subtypes exhibit a distinct geographical distribution, illustrated in Table 3 (worldwide[13,43-51]) and Table 4 (European Regions[10,16,43,52-54]).
Table 3.
The worldwide prevalence of hepatitis C virus genotypes
| Area | The most prevalent genotype | Frequency of other genotypes | First author |
| North America | G1 (80%) | G2 (11.1%) | Thomas et al[13], 2012 |
| 1a- the most common | G3 (7.4%) | ||
| G4 (1.2%) | |||
| Europe | G1 (60%) | G3 (20%); | Messina et al[43], 2015 |
| 1b- the most common | G4 (18%) | ||
| South-East | G3 (65%) | G1 (25%) | Mao et al[44], 2014 |
| Asia | G1 prevails in China, G6 also reported | Li et al[45], 2015 | |
| Middle East | G4 (70%) | G1, G2, G6 | Ray et al[46], 2000 |
| and North Africa | Ramia et al[47], 2012 | ||
| Sub-Saharan and Central Africa | G4 | G5 and G6, G1a, 1b, 2a, 2b | Papastergiou et al[48], 2015 |
| South Africa | G 5 | G1, 2, 3, 4 | Gededzha et al[49], 2014 |
| Asia Pacific and Latin America | G1a | G 1b, 2a, 2b | Messina et al[43], 2015 |
| Ohno et al[50], 1997 | |||
| Villar et al[51], 2015 |
Table 4.
Hepatitis C virus genotypes prevalence in the European regions
| European regions | The most prevalent genotype | Other genotypes | Comments | First author |
| Northern Europe | 1a | 1b, 2, 4 | G1a frequent among PWID | Bruggmann et al[52], 2014 |
| Western Europe | 1b | 3a (France) | G1b-common in older age groups | Messina et al[43], 2015 |
| 4a (United Kingdom, The Netherlands, Germany) | Payan et al[53], 2005 | |||
| Southern Europe | 1b | 2a, 2b, 2c, 4 | G4 is becoming more frequent | Gower et al[10], 2014 |
| Cifuentes et al[54], 2015 | ||||
| Eastern Europe | 1a | 1b, 2, 3, 4 | Non G1 genotypes reported in migrants | Cornberg et al[16], 2011 |
| Messina et al[43], 2015 |
HCV genotype 1 is the most prevalent genotype worldwide; subtype 1a prevails in Northern America, Japan and Northern Europe, and subtype 1b is dominant in Southern Europe and Japan[43,50] and exhibits a high frequency in Northern Africa.
HCV genotype 2 is reported in North America, Japan, Western Africa[55] and Europe (e.g., 2a/c has been isolated in Northern Italy[56] and 2c has been isolated in Southern Italy[57]). Genotype 2a and 1b were identified as the major HCV genotypes circulating in former blood donors from rural China[58].
HCV subtype 3a is endemic in South Eastern Asia, but it is spreading in PWID in United States and Europe, with Germany, France, Italy, and Portugal reporting an increased prevalence of genotypes 1a and 3a[59-62]. Mixed infections have been reported in Italy (1b/3a), Germany (2a/3b), and Sweden (1a/1b)[10].
HCV genotype 4 dominates in the Middle East and Africa. Genotype 4 is responsible for 90% of the nosocomially transmitted HCV infections in Egypt[63] (the country with the highest rate of HCV infections worldwide - 15% of the population, associated with parenteral treatments for schistosomiasis) and most infections in the Democratic Republic of Congo, Central African Republic, Liberia, Uganda, Rwanda and Gabon[64,65]. Infections with genotype 4 are reported with increasing frequency in PWID in Europe.
HCV genotypes 5, 6 and 7 are rather limited in their distribution. The highest prevalence of genotypes 5 and 6 is reported in South Africa[49] and Asia[67], respectively, and genotype 7 was isolated from an emigrant from Congo[68]. A cluster of genotype 5a infections was also recently reported in the Rhodes island of Greece[69].
HCV GENOTYPES CIRCULATING IN PWID
A careful surveillance of circulating genotypes and subtypes is compulsory to reconstruct the natural history of HCV epidemics and viral transmission chains in this high-risk population. Genotypes 1a and 3a predominate in PWID worldwide. Russia and Estonia reported high rates of genotype 3a, especially in young drug users[70,71]. Genotype 3a is also increasing in frequency in Eastern and Central European countries, with growing rates in Bulgaria[72], Serbia and Montenegro[73], Poland[74] and Romania[75]. PWID in England are more likely to harbor genotype 3a relative to other risk groups, in which genotype 1a is prevalent[76].
An increasing proportion of new infections with genotype 4, which predominates in the Middle East and Africa, was identified primarily in Southern European countries, with distinct subtypes prevailing in different geographic regions: 4a in Greece[77], 4d in Italy[78,79], 4c and 4d in Spain[80], and a local spread of subtype 4d in the Netherlands[81]. France reported increased rates of genotype 4 (from 15% in 2003 to 22% in 2012) in persons coinfected with HCV/HIV: PWID and men having sex with men[82].
Table 5 presents the overall prevalence of HCV genotypes in PWID in the most populated countries in Europe.
Table 5.
Hepatitis C virus genotypes prevalence among people who inject drugs in the most populated European countries1
| Country | G1 | G2 | G3 | G4 | G1 + G4 |
| Germany | 63 | 3.8 | 31 | 2.6 | 61 |
| France | 46 | 2.5 | 37 | 9.1 | 55 |
| United Kingdom | 49 | 5.7 | 42 | 0.8 | 50 |
| Italy | 45 | 3.3 | 38 | 13.0 | 58 |
| Spain | 54 | 2.3 | 27 | 16.0 | 69 |
| Poland | 35 | 0.0 | 57 | 8.7 | 44 |
| Romania2 | 73 | 0.0 | 7 | 12.0 | 85 |
| Netherlands | 53 | 6.0 | 32 | 9.0 | 66 |
| Greece2 | 24 | 2.8 | 61 | 11.0 | 36 |
| Sweden | 36 | 8.7 | 34 | 0.9 | 38 |
1Data are adapted from Wiessing et al[34], 2014, with figures for
Greece and Romania, corrected according to more recent estimates, after the recent human immunodeficiency virus/hepatitis C virus outbreaks in people who inject drugs.
FACTORS INVOLVED IN THE DIFFERENT PREVALENCE OF HCV GENOTYPES IN PWID
HCV genotype monitoring allows a real-time insight into the dynamic changes that occur in the global epidemiological picture of HCV infection.
Social vulnerabilities
The spreading of HCV genotypes/subtypes differs significantly between and within countries, between urban and rural settings, and according to the burden of risk-groups and economic status. There is a direct correlation between the gross national income per capita (GNI) and the so-called hepatitis index[83], which represents a comprehensive assessment of public health performances in the handling and treatment of HCV infections (Figure 1). Five main elements compose the hepatitis index: prevention (public awareness), case identification (screening programs), access to treatment (funding and waiting time), treatment outcomes (sustained virological response and adherence to treatment) and the national health strategy[83]. Figure 1 demonstrates that Germany, France and the United Kingdom are the top three performers, whereas the Baltic States, Hungary and Romania exhibit the lowest scores. A national plan for viral hepatitis has been implemented in France, and similar initiatives are ongoing in Scotland, Germany, Bulgaria and Croatia.
Figure 1.
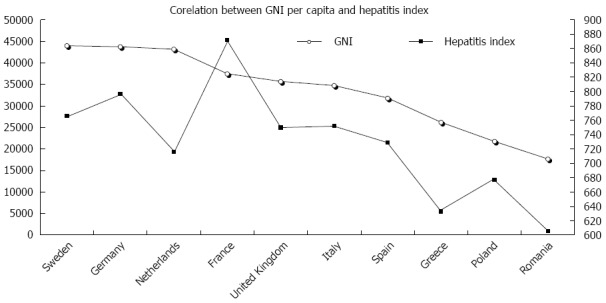
Correlation between the gross national income and the hepatitis index in the top 10 most populated European countries. The gross national income per capita (GNI) directly correlates with the public health performances in the handling and treatment of hepatitis C virus infections (evaluated using the hepatitis index), as calculated by the Euro Hepatitis Report (2012) elaborated by Health Consumer Powerhouse[83].
Case studies: Recent HCV/HIV outbreaks in PWID in Greece and Romania
The impact of the economic crisis on HCV seroprevalence and the distribution of circulating genotypes was recently illustrated by HCV/HIV outbreaks that evolved in PWID in the capital cities of Greece (Athens) and Romania (Bucharest) between 2011 and 2013[84-86]. The gross domestic product per capita in Romania (a country with 20.02 millions inhabitants) and Greece (a country with 11.06 millions inhabitants) is lower than the European Union (EU-27) average (representing only 50 and 75 Purchasing Power Standards, respectively)[87]. Greece has higher unemployment rates than the EU average: 27.3% vs 10.8% of the total labor force, and 58.3% vs 23.4% in persons under 25 years of age. Romania reports a slightly higher unemployment rate in young persons (23.6% in persons aged under 25 years), but a moderate rate of 7.3% in the total labor force[87]. Both countries exhibit higher percentages of people who are at risk of poverty: 22.6% in Romania and 23.1% in Greece relative to the EU average of 17%[33]. The HIV/HCV outbreaks in both countries were associated with financial restrictions in harm-reduction programs, and the persons affected were primarily young males who are unemployed, frequently homeless, and without medical insurances[86,88]. These social vulnerabilities are important triggers for illicit drug use, which increases the associated risk of drug-related infectious diseases and the emergence of different genotypes than the genotypes circulating in the general population. HCV genotype 1b[89] and HIV subtype F[90] predominate in Romania, but the introduction of new viral strains was documented during a recent outbreak in PWID: HCV subtypes 1a, 3a, 4 (Ruta S, unpublished data) and HIV subtype G, with the particular recombinant form CRF14_BG[90]. HCV genotype 3[91] and HIV CRF14_BG and CRF_35AD[92] prevail in PWID in Greece. Assessments of the evolution of HCV infection in older patients (infected with genotype 1, primarily through nosocomial procedures) vs younger patients (infected with newly introduced genotypes, primarily through IDU) will be interesting. Younger patients are candidates for shorter durations of therapy, with important implications for treatment-related costs and patient quality of life.
Immigration from HCV endemic countries and the evolving networks of HCV transmission in PWID influence the genotype distribution. European countries with the highest number of migrants (Germany: 12.3%, Italy, Spain, Netherlands: each 10%-12%, and France: 10%)[93] exhibit a high prevalence of HCV infection and increased frequencies of less common genotypes. One recent study demonstrated that more than one third of the patients with chronic hepatitis C from Germany were born abroad[13], and an increased prevalence of HCV infection was reported in migrants in Italy[94]. Many cases of HCV infection in PWID from Cyprus are diagnosed in foreign nationals[95]. The increasing prevalence of non-1b genotypes in France, Spain, Italy and Greece was primarily attributed to a large flow of immigrants, but some limited molecular epidemiology studies argue against this hypothesis[96,97].
Phylogenetic analyses recently identified HCV transmission clusters associated with injection relationships in Melbourne, Australia[98] and Vancouver, Canada[99].
WHAT ARE THE CONSEQUENCES OF THE DISTINCT PREVALENCE OF HCV GENOTYPES IN HIGH-RISK POPULATIONS?
HCV variability triggers important clinical consequences. The emergence of immune response escape mutants accounts for the high level of chronic infection, and the infecting genotype is critical for the natural and on-treatment evolution of the infection. These data are especially significant for PWID, who are frequently infected with genotypes 1a, 3 and 4 that tend to exhibit less favorable responses to therapies, as discussed below.
Interferon-based therapy
HCV genotype was one of the primary predictors of the response rate to the classic pegylated interferon-ribavirin (P/R) therapy, which is the only affordable therapy in developing countries. HCV subtype 1b exhibits the most unfavorable response profile, and genotypes 2 and 3 are “easy-to-treat” and exhibit a sustained virological response (SVR) in up to 80% of treated patients[100]. The reported SVR rates for genotype 4 are 60%-69% in Egypt and 40%-50% in countries outside endemic areas[64]. Genotype 3, initially correlated with a very high response rate to the classic P/R treatment, is associated with a higher rate of liver fibrosis and steatosis (unlinked to insulin resistance) and a more rapidly progressive end-stage liver disease[101]. Subsequently, many genotype 3-infected patients, including PWID, exhibit cirrhosis at the initiation of P/R treatment, and the overall response rate has been disappointing.
Direct-acting antiviral-based regimens
Treatment regimens for chronic hepatitis C and the inclusion criteria have largely changed in the last 4 years with the approval of new DAAs. However, the HCV genotype matters for therapeutic responses[102]. Novel treatments for HCV are highly cost-effective for HCV genotype 1. The current World Health Organization[103], American Association for the Study of Liver[104] and European Association for the Study of Liver[105] guidelines for HCV treatment are genotype-dependent, with several available options for each genotype, including IFN-free regimens, considered the most suitable ones in genotype 2-infected patients, and recommended for genotypes 1, 3 and 4. However, the triple combination of pegylated IFN-α, ribavirin and sofosbuvir (a NS5B inhibitor) administered for 12 wk is still favored in terms of efficacy, for patients infected with HCV genotypes 1, 3, 4, 5 and 6, as well as for those infected with genotype 2 that are cirrhotic and/or treatment-experienced[105,106]. This regimen also avoids resistance selection in cases of treatment failure. A combination of sofosbuvir and ledipasvir (an NS5A inhibitor), administered as a single pill, is currently recommended by the AASL as a first-line agent for patients without cirrhosis[104,107]. The new DAAs are less effective for patients infected with genotype 3 who have advanced liver disease, which is frequently observed in PWID. Phase III clinical studies of sofosbuvir and ribavirin revealed a sustained virological response in only 60% of patients with genotype 3 and cirrhosis who had previously failed P/R treatment, even in the case of a longer therapy duration[108,109]. Even the newly approved NS5A inhibitors, including ledipasvir, are less active against HCV genotype 3 than against other genotypes[110-112]. Therefore, genotype 3, which is prevalent in PWID, is currently considered one of the “difficult to treat” genotypes. Few studies have addressed the efficacy of the new oral regimens in patients infected with HCV genotypes 4, 5, and 6[48,113], which are less prevalent in Europe and North America.
The impact of HCV genotype on the development of viral resistance
Viral breakthrough during or after DAA treatment (especially with the first generation protease inhibitors, telaprevir and boceprevir) was associated with the selection of resistance-associated variants, which preexist as minority populations[114-116]. Differences in the genetic barrier to resistance exist between subtypes; resistance mutations arise more quickly in patients who are infected with genotype 1a[115,116]. Moreover, a series of natural HCV polymorphisms that are found with different frequencies according to the HCV subtype, can influence treatment outcomes[117]. A second generation protease inhibitor (Simeprevir) exhibits reduced efficacy on subtype 1a strains because of the high prevalence of a specific mutation (Q80K) at baseline[118,119]. The activity of an NS5 inhibitor, which was recently approved for the treatment of HCV infection (Daclatasvir), is inhibited in the presence of a natural polymorphism (Q30R), which is found in more than 50% of genotype 4 strains[117,120]. Notably, resistant HCV variants are not archived (because HCV, unlike HIV or HBV, does not establish reservoirs), and reversion to the wild-type strain is observed 10-29 mo after treatment interruption[121] (faster for subtype 1b compared with 1a[114]). These differences are likely important for the treatment of PWID.
Toward a patient-tailored therapy for chronic hepatitis C in PWID
Important barriers to care and treatment are present in vulnerable populations, such as PWID[122], and it is estimated that only one in ten diagnosed patients enter treatment for hepatitis C. Delays in diagnosis lead to late presentations, with associated high viral loads and significant fibrosis, that represent unfavorable predictors for treatment efficacy. Decisions to treat are taken on a case-by-case basis, and treatments are accompanied by active counseling to decrease or cease drug and alcohol intake and the promotion of comprehensive harm-reduction programs, including in prisons[123-125].
The same therapeutic regimens based on DAAs are recommended for PWID, and a history of drug use or recent drug use is not associated with a reduced response rate[105]. The perceived risk of reinfection is not a reason for treatment denial, but instead a possibility that must be actively monitored after the achievement of SVR. The estimated rate of reinfection in PWID with persistent risk behaviors following successful HCV treatment is approximately 1%-5%[126-128].
However, the prohibitive costs of highly efficient therapeutic DAA options have prevented their use outside countries with high incomes. The NS5B polymerase inhibitor Sofosbuvir costs $1000 per day, the combination of sofosbuvir with ledipasvir costs $1125 per day, and a short 12-wk IFN-free regimen can reach a price of $150000 per patient[129]. Therefore, many countries that have high seroprevalence rates of HCV infection in the general population and vulnerable risk groups, continue to rely on the classic dual P/R therapy or triple therapy that combines P/R with first-generation protease inhibitors.
Changes in circulating genotypes suggest the necessity of different clinical approaches, including the choice of the most suitable and cost-effective antiviral combination therapy for patients who are “difficult to reach, manage and treat”[130]. Therefore, the deferral of a P/R-based treatment that has several challenges (e.g., administration, monitoring and management of related side-effects), may be an option for patients with an early fibrosis stage[131], while waiting for highly effective, pangenotypic-active combinations to become available at more reasonable prices in the foreseeable future. Other factors will likely influence the final decision, including: the cost-effectiveness of the IFN-free regimens (treatment duration plotted against the SVR rate), the adherence to treatment and the cumulative toxicities (which are important factors in PWID, especially in HIV-coinfected patients), and the extent of clinically relevant viral resistance[131-134].
CONCLUSION
Many PWID who are infected with HCV remain undiagnosed. The distribution of circulating genotypes in this vulnerable group is distinct from the general population. Transmission networks associated with drug use, increased global travel and immigration are the primary factors behind this different epidemiological picture. PWID are critical epidemiological connectors to the general populations and drug use is a key vector for the diversification of circulating viral genotypes. The determination of circulating HCV genotypes in high-risk groups, such as PWID, who frequently have additional risk factors (poverty, imprisonment, and HIV coinfections) will provide a further understanding of the global viral epidemiology. HCV genetic diversity has a major impact on viral persistence, evolution to cirrhosis and hepatocellular carcinoma and potential resistance to antiviral agents. Therefore, knowledge of HCV genotypes will likely remain an essential factor for the correct design of national health programs, even with the introduction of new antivirals.
ACKNOWLEDGMENTS
The authors thank Claudia Dita, MD from the Stefan S. Nicolau Institute of Virology, Bucharest, for technical support.
Footnotes
Supported by NIH grant 5P30 AI036211-19 (partially, subcontract PO 5600167489, through the Baylor International Pediatric AIDS Initiative); and the Romanian Academy Programme for the Stefan S. Nicolau Institute of Virology.
Conflict-of-interest statement: We undersigned, Simona Ruta and Costin Cernescu, authors of the review entitled “Injecting drug use: A vector for introduction of new hepatitis C virus genotypes”, submitted to World Journal of Gastroenterology declare no competing commercial, personal, political, intellectual, or religious interests in relation to the submitted work.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: April 29, 2015
First decision: June 2, 2015
Article in press: September 15, 2015
P- Reviewer: Chuang WL S- Editor: Yu J L- Editor: A E- Editor: Ma S
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 4.Cooke GS, Lemoine M, Thursz M, Gore C, Swan T, Kamarulzaman A, DuCros P, Ford N. Viral hepatitis and the Global Burden of Disease: a need to regroup. J Viral Hepat. 2013;20:600–601. doi: 10.1111/jvh.12123. [DOI] [PubMed] [Google Scholar]
- 5.Amin J, Law MG, Bartlett M, Kaldor JM, Dore GJ. Causes of death after diagnosis of hepatitis B or hepatitis C infection: a large community-based linkage study. Lancet. 2006;368:938–945. doi: 10.1016/S0140-6736(06)69374-4. [DOI] [PubMed] [Google Scholar]
- 6.Quan PL, Firth C, Conte JM, Williams SH, Zambrana-Torrelio CM, Anthony SJ, Ellison JA, Gilbert AT, Kuzmin IV, Niezgoda M, et al. Bats are a major natural reservoir for hepaciviruses and pegiviruses. Proc Natl Acad Sci USA. 2013;110:8194–8199. doi: 10.1073/pnas.1303037110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapoor A, Simmonds P, Scheel TK, Hjelle B, Cullen JM, Burbelo PD, Chauhan LV, Duraisamy R, Sanchez Leon M, Jain K, et al. Identification of rodent homologs of hepatitis C virus and pegiviruses. MBio. 2013;4:e00216–e00213. doi: 10.1128/mBio.00216-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nat Rev Microbiol. 2007;5:453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- 9.Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59:318–327. doi: 10.1002/hep.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol. 2014;61:S45–S57. doi: 10.1016/j.jhep.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 11.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–2441. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.United Nations Office on Drugs and Crime. World Drug Report 2014. United Nations Publications, eISBN: 978-92-1-056752-7. Available from: http://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf.
- 13.Thomas LB, Foulis PR, Mastorides SM, Djilan YA, Skinner O, Borkowski AA. Hepatitis C genotype analysis: results in a large veteran population with review of the implications for clinical practice. Ann Clin Lab Sci. 2012;42:355–362. [PubMed] [Google Scholar]
- 14.Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008;48:148–162. doi: 10.1016/j.jhep.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 15.European Centre for Disease Prevention and Control. Hepatitis B and C surveillance in Europe, 2012. Stockholm: ECDC; 2014. pp. TQ–AU-14-001-EN-N. Available from: http://ecdc.europa.eu/en/hepatitis-b-c-surveillance-europe-2012-july-2014.pdf. [Google Scholar]
- 16.Cornberg M, Razavi HA, Alberti A, Bernasconi E, Buti M, Cooper C, Dalgard O, Dillion JF, Flisiak R, Forns X, et al. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 2011;31 Suppl 2:30–60. doi: 10.1111/j.1478-3231.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 17.Mathurin P. HCV burden in Europe and the possible impact of current treatment. Dig Liver Dis. 2013;45 Suppl 5:S314–S317. doi: 10.1016/j.dld.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 18.McGarry LJ, Pawar VS, Panchmatia HR, Rubin JL, Davis GL, Younossi ZM, Capretta JC, O’Grady MJ, Weinstein MC. Economic model of a birth cohort screening program for hepatitis C virus. Hepatology. 2012;55:1344–1355. doi: 10.1002/hep.25510. [DOI] [PubMed] [Google Scholar]
- 19.Kabiri M, Jazwinski AB, Roberts MS, Schaefer AJ, Chhatwal J. The changing burden of hepatitis C virus infection in the United States: model-based predictions. Ann Intern Med. 2014;161:170–180. doi: 10.7326/M14-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis H, Burke K, Begum S, Ushiro-Limb I, Foster G. What is the best method of case finding for chronic viral Hepatitis in at-risk migrant communities? J Hepatology. 2012;56:S351. [Google Scholar]
- 21.Litwin AH, Smith BD, Drainoni ML, McKee D, Gifford AL, Koppelman E, Christiansen CL, Weinbaum CM, Southern WN. Primary care-based interventions are associated with increases in hepatitis C virus testing for patients at risk. Dig Liver Dis. 2012;44:497–503. doi: 10.1016/j.dld.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Hahné SJ, Veldhuijzen IK, Wiessing L, Lim TA, Salminen M, Laar Mv. Infection with hepatitis B and C virus in Europe: a systematic review of prevalence and cost-effectiveness of screening. BMC Infect Dis. 2013;13:181. doi: 10.1186/1471-2334-13-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Degenhardt L, Whiteford HA, Ferrari AJ, Baxter AJ, Charlson FJ, Hall WD, Freedman G, Burstein R, Johns N, Engell RE, et al. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1564–1574. doi: 10.1016/S0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. A strategy to halt and reverse the HIV epidemic among people who inject drugs in Asia and the Pacific: 2010–2015, WHO Library Cataloguing 2015. Available from: http://iris.wpro.who.int/handle/10665.1/5506.
- 25.Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, Degenhardt L. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 27.Page K, Morris MD, Hahn JA, Maher L, Prins M. Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clin Infect Dis. 2013;57 Suppl 2:S32–S38. doi: 10.1093/cid/cit300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tseng FC, O’Brien TR, Zhang M, Kral AH, Ortiz-Conde BA, Lorvick J, Busch MP, Edlin BR. Seroprevalence of hepatitis C virus and hepatitis B virus among San Francisco injection drug users, 1998 to 2000. Hepatology. 2007;46:666–671. doi: 10.1002/hep.21765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 30.Mühlberger N, Schwarzer R, Lettmeier B, Sroczynski G, Zeuzem S, Siebert U. HCV-related burden of disease in Europe: a systematic assessment of incidence, prevalence, morbidity, and mortality. BMC Public Health. 2009;9:34. doi: 10.1186/1471-2458-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarna A, Panda S. HCV in people who inject drugs: a neglected epidemic. Lancet Infect Dis. 2015;15:4–5. doi: 10.1016/S1473-3099(14)71054-0. [DOI] [PubMed] [Google Scholar]
- 32.Hope VD, Eramova I, Capurro D, Donoghoe MC. Prevalence and estimation of hepatitis B and C infections in the WHO European Region: a review of data focusing on the countries outside the European Union and the European Free Trade Association. Epidemiol Infect. 2014;142:270–286. doi: 10.1017/S0950268813000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) European Drug Report. Trends and developments 2014. Luxembourg: Publications Office of the European Union; 2014. Available from: http://www.ab.gov.tr/files/ardb/evt/european_drug_report_2014.pdf. [Google Scholar]
- 34.Wiessing L, Ferri M, Grady B, Kantzanou M, Sperle I, Cullen KJ, Hatzakis A, Prins M, Vickerman P, Lazarus JV, et al. Hepatitis C virus infection epidemiology among people who inject drugs in Europe: a systematic review of data for scaling up treatment and prevention. PLoS One. 2014;9:e103345. doi: 10.1371/journal.pone.0103345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pilon R, Leonard L, Kim J, Vallee D, De Rubeis E, Jolly AM, Wylie J, Pelude L, Sandstrom P. Transmission patterns of HIV and hepatitis C virus among networks of people who inject drugs. PLoS One. 2011;6:e22245. doi: 10.1371/journal.pone.0022245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vickerman P, Martin NK, Roy A, Beattie T, Jarlais DD, Strathdee S, Wiessing L, Hickman M. Is the HCV-HIV co-infection prevalence amongst injecting drug users a marker for the level of sexual and injection related HIV transmission? Drug Alcohol Depend. 2013;132:172–181. doi: 10.1016/j.drugalcdep.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Wiessing L, Likatavicius G, Hedrich D, Guarita B, van de Laar MJ, Vicente J. Trends in HIV and hepatitis C virus infections among injecting drug users in Europe, 2005 to 2010. Euro Surveill. 2011;16:pii 20031. [PubMed] [Google Scholar]
- 38.Lidman C, Norden L, Kåberg M, Käll K, Franck J, Aleman S, Birk M. Hepatitis C infection among injection drug users in Stockholm Sweden: prevalence and gender. Scand J Infect Dis. 2009;41:679–684. doi: 10.1080/00365540903062143. [DOI] [PubMed] [Google Scholar]
- 39.García-Fulgueiras A, García-Pina R, Morant C, de Larrea-Baz NF, Alvarez E. Burden of disease related to hepatitis C and hepatitis B in Spain: a methodological challenge of an unfolding health problem. J Viral Hepat. 2011;18:e453–e460. doi: 10.1111/j.1365-2893.2011.01467.x. [DOI] [PubMed] [Google Scholar]
- 40.Vermehren J, Schlosser B, Domke D, Elanjimattom S, Müller C, Hintereder G, Hensel-Wiegel K, Tauber R, Berger A, Haas N, et al. High prevalence of anti-HCV antibodies in two metropolitan emergency departments in Germany: a prospective screening analysis of 28,809 patients. PLoS One. 2012;7:e41206. doi: 10.1371/journal.pone.0041206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flisiak R, Halota W, Tomasiewicz K, Kostrzewska K, Razavi HA, Gower EE. Forecasting the disease burden of chronic hepatitis C virus in Poland. Eur J Gastroenterol Hepatol. 2015;27:70–76. doi: 10.1097/MEG.0000000000000237. [DOI] [PubMed] [Google Scholar]
- 42.Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mao XR, Zhang LT, Chen H, Xiao P, Zhang YC. Correlation between the genetic variations in interleukin 28B and chronic hepatitis C virus genotypes in the Chinese population. Mol Med Rep. 2014;10:1037–1045. doi: 10.3892/mmr.2014.2242. [DOI] [PubMed] [Google Scholar]
- 45.Li C, Barnes E, Newton PN, Fu Y, Vongsouvath M, Klenerman P, Okamoto H, Abe K, Pybus OG, Lu L. An expanded taxonomy of hepatitis C virus genotype 6: Characterization of 22 new full-length viral genomes. Virology. 2015;476:355–363. doi: 10.1016/j.virol.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ray SC, Arthur RR, Carella A, Bukh J, Thomas DL. Genetic epidemiology of hepatitis C virus throughout egypt. J Infect Dis. 2000;182:698–707. doi: 10.1086/315786. [DOI] [PubMed] [Google Scholar]
- 47.Ramia S, Melhem NM, Kreidieh K. Hepatitis C virus infection in the Middle East and North Africa “MENA” region: injecting drug users (IDUs) is an under-investigated population. Infection. 2012;40:1–10. doi: 10.1007/s15010-011-0236-z. [DOI] [PubMed] [Google Scholar]
- 48.Papastergiou V, Karatapanis S. Current status and emerging challenges in the treatment of hepatitis C virus genotypes 4 to 6. World J Clin Cases. 2015;3:210–220. doi: 10.12998/wjcc.v3.i3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gededzha MP, Selabe SG, Blackard JT, Kyaw T, Mphahlele MJ. Near full-length genome analysis of HCV genotype 5 strains from South Africa. Infect Genet Evol. 2014;21:118–123. doi: 10.1016/j.meegid.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 50.Ohno O, Mizokami M, Wu RR, Saleh MG, Ohba K, Orito E, Mukaide M, Williams R, Lau JY. New hepatitis C virus (HCV) genotyping system that allows for identification of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and 6a. J Clin Microbiol. 1997;35:201–207. doi: 10.1128/jcm.35.1.201-207.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Villar LM, Ó KM, Scalioni LP, Cruz HM, Portilho MM, Mendonça AC, Miguel JC, Figueiredo AS, Almeida AJ, Lampe E. Prevalence of hepatitis B and C virus infections among military personnel. Braz J Infect Dis. 2015;19:285–290. doi: 10.1016/j.bjid.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruggmann P, Berg T, Øvrehus AL, Moreno C, Brandão Mello CE, Roudot-Thoraval F, Marinho RT, Sherman M, Ryder SD, Sperl J, et al. Historical epidemiology of hepatitis C virus (HCV) in selected countries. J Viral Hepat. 2014;21 Suppl 1:5–33. doi: 10.1111/jvh.12247. [DOI] [PubMed] [Google Scholar]
- 53.Payan C, Roudot-Thoraval F, Marcellin P, Bled N, Duverlie G, Fouchard-Hubert I, Trimoulet P, Couzigou P, Cointe D, Chaput C, et al. Changing of hepatitis C virus genotype patterns in France at the beginning of the third millenium: The GEMHEP GenoCII Study. J Viral Hepat. 2005;12:405–413. doi: 10.1111/j.1365-2893.2005.00605.x. [DOI] [PubMed] [Google Scholar]
- 54.Cifuentes C, Mancebo-Hernández M, Pérez-Navarro E, Recio E, Monje-Agudo P, Valiente A, Pineda JA. [Prevalence and genotype distribution changes in hepatitis C virus co-infection among human immunodeficiency virus-infected patients] Enferm Infecc Microbiol Clin. 2015;33:110–112. doi: 10.1016/j.eimc.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 55.Candotti D, Temple J, Sarkodie F, Allain JP. Frequent recovery and broad genotype 2 diversity characterize hepatitis C virus infection in Ghana, West Africa. J Virol. 2003;77:7914–7923. doi: 10.1128/JVI.77.14.7914-7923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osella AR, Misciagna G, Guerra V, Elba S, Buongiorno G, Cavallini A, Di Leo A, Sonzogni L, Mondelli MU, Silini EM. Hepatitis C virus genotypes and risk of cirrhosis in southern Italy. Clin Infect Dis. 2001;33:70–75. doi: 10.1086/320887. [DOI] [PubMed] [Google Scholar]
- 57.Ansaldi F, Bruzzone B, Salmaso S, Rota MC, Durando P, Gasparini R, Icardi G. Different seroprevalence and molecular epidemiology patterns of hepatitis C virus infection in Italy. J Med Virol. 2005;76:327–332. doi: 10.1002/jmv.20376. [DOI] [PubMed] [Google Scholar]
- 58.Yin W, Huang C, Qiu F, Liu L, Wang F, Zhou J, Zhang Y, Bi S. Risk factors of hepatitis C virus transmission and genotype distribution in former blood donors from Chinese rural area. BMC Public Health. 2015;15:184. doi: 10.1186/s12889-015-1535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morice Y, Cantaloube JF, Beaucourt S, Barbotte L, De Gendt S, Goncales FL, Butterworth L, Cooksley G, Gish RG, Beaugrand M, et al. Molecular epidemiology of hepatitis C virus subtype 3a in injecting drug users. J Med Virol. 2006;78:1296–1303. doi: 10.1002/jmv.20692. [DOI] [PubMed] [Google Scholar]
- 60.Stroffolini T, Fiumeb A, Fataleb G, Regnib F, Ciccozzia M, Marzolinia A, Mele A. Hepatitis C virus among intravenous drug users in Italy. Hepatology Research. 1997;9:20–27. [Google Scholar]
- 61.Calado RA, Rocha MR, Parreira R, Piedade J, Venenno T, Esteves A. Hepatitis C virus subtypes circulating among intravenous drug users in Lisbon, Portugal. J Med Virol. 2011;83:608–615. doi: 10.1002/jmv.21955. [DOI] [PubMed] [Google Scholar]
- 62.Sereno S, Perinelli P, Laghi V. Changes in the prevalence of hepatitis C virus genotype among Italian injection drug users-relation to period of injection started. J Clin Virol. 2009;45:354–357. doi: 10.1016/j.jcv.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 63.Mohamoud YA, Mumtaz GR, Riome S, Miller D, Abu-Raddad LJ. The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis. BMC Infect Dis. 2013;13:288. doi: 10.1186/1471-2334-13-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kamal SM, Nasser IA. Hepatitis C genotype 4: What we know and what we don’t yet know. Hepatology. 2008;47:1371–1383. doi: 10.1002/hep.22127. [DOI] [PubMed] [Google Scholar]
- 65.Xu LZ, Larzul D, Delaporte E, Bréchot C, Kremsdorf D. Hepatitis C virus genotype 4 is highly prevalent in central Africa (Gabon) J Gen Virol. 1994;75(Pt 9):2393–2398. doi: 10.1099/0022-1317-75-9-2393. [DOI] [PubMed] [Google Scholar]
- 66.Iles JC, Raghwani J, Harrison GL, Pepin J, Djoko CF, Tamoufe U, LeBreton M, Schneider BS, Fair JN, Tshala FM, et al. Phylogeography and epidemic history of hepatitis C virus genotype 4 in Africa. Virology. 2014;464-465:233–243. doi: 10.1016/j.virol.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thong VD, Akkarathamrongsin S, Poovorawan K, Tangkijvanich P, Poovorawan Y. Hepatitis C virus genotype 6: virology, epidemiology, genetic variation and clinical implication. World J Gastroenterol. 2014;20:2927–2940. doi: 10.3748/wjg.v20.i11.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murphy DG, Willems B, Deschênes M, Hilzenrat N, Mousseau R, Sabbah S. Use of sequence analysis of the NS5B region for routine genotyping of hepatitis C virus with reference to C/E1 and 5’ untranslated region sequences. J Clin Microbiol. 2007;45:1102–1112. doi: 10.1128/JCM.02366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karatapanis S, Tsoplou P, Papastergiou V, Vasiageorgi A, Stampori M, Saitis I, Tsitsopoulos E, Lisgos P, Skorda L, Ketikoglou I, et al. Hepatitis C virus genotyping in Greece: unexpected high prevalence of genotype 5a in a Greek island. J Med Virol. 2012;84:223–228. doi: 10.1002/jmv.22249. [DOI] [PubMed] [Google Scholar]
- 70.Paintsil E, Verevochkin SV, Dukhovlinova E, Niccolai L, Barbour R, White E, Toussova OV, Alexander L, Kozlov AP, Heimer R. Hepatitis C virus infection among drug injectors in St Petersburg, Russia: social and molecular epidemiology of an endemic infection. Addiction. 2009;104:1881–1890. doi: 10.1111/j.1360-0443.2009.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tallo T, Norder H, Tefanova V, Krispin T, Schmidt J, Ilmoja M, Orgulas K, Pruunsild K, Priimägi L, Magnius LO. Genetic characterization of hepatitis C virus strains in Estonia: fluctuations in the predominating subtype with time. J Med Virol. 2007;79:374–382. doi: 10.1002/jmv.20828. [DOI] [PubMed] [Google Scholar]
- 72.Ciccozzi M, Zehender G, Cento V, Lo Presti A, Teoharov P, Pavlov I, Bogdanova V, Perno CF, Ciotti M. Molecular analysis of hepatitis C virus infection in Bulgarian injecting drug users. J Med Virol. 2011;83:1565–1570. doi: 10.1002/jmv.22154. [DOI] [PubMed] [Google Scholar]
- 73.Svirtlih N, Delic D, Simonovic J, Jevtovic D, Dokic L, Gvozdenovic E, Boricic I, Terzic D, Pavic S, Neskovic G, et al. Hepatitis C virus genotypes in Serbia and Montenegro: the prevalence and clinical significance. World J Gastroenterol. 2007;13:355–360. doi: 10.3748/wjg.v13.i3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chlabicz S, Flisiak R, Kowalczuk O, Grzeszczuk A, Pytel-Krolczuk B, Prokopowicz D, Chyczewski L. Changing HCV genotypes distribution in Poland--relation to source and time of infection. J Clin Virol. 2008;42:156–159. doi: 10.1016/j.jcv.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 75.Sultana C, Vagu C, Temereanca A, Grancea C, Slobozeanu J, Ruta S. Hepatitis C Virus Genotypes in Injecting Drug Users from Romania. Cent Eur J Med. 2011;6:672–678. doi: 10.2478/s11536-011-0073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.May S, Ngui SL, Collins S, Lattimore S, Ramsay M, Tedder RS, Ijaz S. Molecular epidemiology of newly acquired hepatitis C infections in England 2008-2011: genotype, phylogeny and mutation analysis. J Clin Virol. 2015;64:6–11. doi: 10.1016/j.jcv.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 77.Katsoulidou A, Sypsa V, Tassopoulos NC, Boletis J, Karafoulidou A, Ketikoglou I, Tsantoulas D, Vafiadi I, Hatzis G, Skoutelis A, et al. Molecular epidemiology of hepatitis C virus (HCV) in Greece: temporal trends in HCV genotype-specific incidence and molecular characterization of genotype 4 isolates. J Viral Hepat. 2006;13:19–27. doi: 10.1111/j.1365-2893.2005.00649.x. [DOI] [PubMed] [Google Scholar]
- 78.Matera G, Lamberti A, Quirino A, Focà D, Giancotti A, Barreca GS, Guadagnino V, Liberto MC. Changes in the prevalence of hepatitis C virus (HCV) genotype 4 in Calabria, Southern Italy. Diagn Microbiol Infect Dis. 2002;42:169–173. doi: 10.1016/s0732-8893(01)00350-9. [DOI] [PubMed] [Google Scholar]
- 79.Liberto MC, Marascio N, Zicca E, Matera G. Epidemiological features and specificities of HCV infection: a hospital-based cohort study in a university medical center of Calabria region. BMC Infect Dis. 2012;12 Suppl 2:S4. doi: 10.1186/1471-2334-12-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sánchez-Quijano A, Abad MA, Torronteras R, Rey C, Pineda JA, Leal M, Macias J, Lissen E. Unexpected high prevalence of hepatitis C virus genotype 4 in Southern Spain. J Hepatol. 1997;27:25–29. doi: 10.1016/s0168-8278(97)80275-9. [DOI] [PubMed] [Google Scholar]
- 81.de Bruijne J, Schinkel J, Prins M, Koekkoek SM, Aronson SJ, van Ballegooijen MW, Reesink HW, Molenkamp R, van de Laar TJ. Emergence of hepatitis C virus genotype 4: phylogenetic analysis reveals three distinct epidemiological profiles. J Clin Microbiol. 2009;47:3832–3838. doi: 10.1128/JCM.01146-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cacoub P, Dabis F, Costagliola D, Almeida K, Lert F, Piroth L, Semaille C. Burden of HIV and hepatitis C co-infection: the changing epidemiology of hepatitis C in HIV-infected patients in France. Liver Int. 2015;35:65–70. doi: 10.1111/liv.12639. [DOI] [PubMed] [Google Scholar]
- 83.Cebolla B, Björnberg A, editors. Health Consumer Powerhouse: Euro Hepatitis Index Report. 2012. Available from: www.healthpowerhouse.com.
- 84.Pharris A, Wiessing L, Sfetcu O, Hedrich D, Botescu A, Fotiou A, Nikolopoulos GK, Malliori M, Salminen M, Suk JE, et al. Human immunodeficiency virus in injecting drug users in Europe following a reported increase of cases in Greece and Romania, 2011. Euro Surveill. 2011;16:pii 20032. [PubMed] [Google Scholar]
- 85.Paraskevis D, Nikolopoulos G, Tsiara C, Paraskeva D, Antoniadou A, Lazanas M, Gargalianos P, Psychogiou M, Malliori M, Kremastinou J, et al. HIV-1 outbreak among injecting drug users in Greece, 2011: a preliminary report. Euro Surveill. 2011;16:pii 19962. doi: 10.2807/ese.16.36.19962-en. [DOI] [PubMed] [Google Scholar]
- 86.Oprea C, Ceausu E, Ruta S. Ongoing outbreak of multiple blood-borne infections in injecting drug users in Romania. Public Health. 2013;127:1048–1050. doi: 10.1016/j.puhe.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 87.European Commision. Eurostat Yearbook, 2014. Available from: http://ec.europa.eu/eurostat.
- 88.Sypsa V, Paraskevis D, Malliori M, Nikolopoulos GK, Panopoulos A, Kantzanou M, Katsoulidou A, Psichogiou M, Fotiou A, Pharris A, et al. Homelessness and Other Risk Factors for HIV Infection in the Current Outbreak Among Injection Drug Users in Athens, Greece. Am J Public Health. 2015;105:196–204. doi: 10.2105/AJPH.2013.301656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sultana C, Oprisan G, Szmal C, Vagu C, Temereanca A, Dinu S, Teleman MD, Ruta S. Molecular epidemiology of hepatitis C virus strains from Romania. J Gastrointestin Liver Dis. 2011;20:261–266. [PubMed] [Google Scholar]
- 90.Niculescu I, Paraschiv S, Paraskevis D, Abagiu A, Batan I, Banica L, Otelea D. Recent HIV-1 Outbreak Among Intravenous Drug Users in Romania: Evidence for Cocirculation of CRF14_BG and Subtype F1 Strains. AIDS Res Hum Retroviruses. 2015;31:488–495. doi: 10.1089/aid.2014.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gigi E, Sinakos E, Sykja A, Androulakis G, Tanis C, Stayridou V, Tsirogianni E, Zouridakis K, Bellou AL, Orfanou E, et al. Epidemiology, clinical data, and treatment of viral hepatitis in a large cohort of intravenous drug users. J Addict Med. 2013;7:52–57. doi: 10.1097/ADM.0b013e318279756f. [DOI] [PubMed] [Google Scholar]
- 92.Paraskevis D, Nikolopoulos G, Fotiou A, Tsiara C, Paraskeva D, Sypsa V, Lazanas M, Gargalianos P, Psichogiou M, Skoutelis A, et al. Economic recession and emergence of an HIV-1 outbreak among drug injectors in Athens metropolitan area: a longitudinal study. PLoS One. 2013;8:e78941. doi: 10.1371/journal.pone.0078941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.International Organization for Migration. The World Migration Report 2013: Migrant Well-being and Development - the 7th report in IOM’s World Migration Report series, 2013, Geneva. Available from: http://www.iom.int.
- 94.Marascio N, Liberto M, Barreca G, Zicca E, Quirino A, Lamberti A, Bianco G, Matera G, Surace L, Berardelli G, et al. Update on epidemiology of HCV in Italy: focus on the Calabria Region. BMC Infect Dis. 2014;14 Suppl 5:S2. doi: 10.1186/1471-2334-14-S5-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zehender G, Sorrentino C, Lai A, Ebranati E, Gabanelli E, Lo Presti A, Vujoševic D, Lauševic D, Terzić D, Shkjezi R, et al. Reconstruction of the evolutionary dynamics of hepatitis C virus subtypes in Montenegro and the Balkan region. Infect Genet Evol. 2013;17:223–230. doi: 10.1016/j.meegid.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 96.Di Lello FA, Neukam K, Parra-Sanchez M, Plaza Z, Soriano V, Cifuentes C, Mira JA, Poveda E, Pineda JA. Hepatitis C virus genotype 4 in Southern and Central Spain does not originate from recent foreign migration waves. J Med Virol. 2013;85:1734–1740. doi: 10.1002/jmv.23657. [DOI] [PubMed] [Google Scholar]
- 97.Ciccozzi M, Equestre M, Costantino A, Marascio N, Quirino A, Lo Presti A, Cella E, Bruni R, Liberto MC, Focà A, et al. Hepatitis C virus genotype 4d in Southern Italy: reconstruction of its origin and spread by a phylodynamic analysis. J Med Virol. 2012;84:1613–1619. doi: 10.1002/jmv.23384. [DOI] [PubMed] [Google Scholar]
- 98.Sacks-Davis R, Daraganova G, Aitken C, Higgs P, Tracy L, Bowden S, Jenkinson R, Rolls D, Pattison P, Robins G, et al. Hepatitis C virus phylogenetic clustering is associated with the social-injecting network in a cohort of people who inject drugs. PLoS One. 2012;7:e47335. doi: 10.1371/journal.pone.0047335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jacka B, Applegate T, Krajden M, Olmstead A, Harrigan PR, Marshall BD, DeBeck K, Milloy MJ, Lamoury F, Pybus OG, et al. Phylogenetic clustering of hepatitis C virus among people who inject drugs in Vancouver, Canada. Hepatology. 2014;60:1571–1580. doi: 10.1002/hep.27310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lim SG. Chronic hepatitis C genotype 1 treatment roadmap for resource constrained settings. World J Gastroenterol. 2015;21:1972–1981. doi: 10.3748/wjg.v21.i6.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abenavoli L, Masarone M, Peta V, Milic N, Kobyliak N, Rouabhia S, Persico M. Insulin resistance and liver steatosis in chronic hepatitis C infection genotype 3. World J Gastroenterol. 2014;20:15233–15240. doi: 10.3748/wjg.v20.i41.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Quer J, Gregori J, Rodríguez-Frias F, Buti M, Madejon A, Perez-del-Pulgar S, Garcia-Cehic D, Casillas R, Blasi M, Homs M, et al. High-resolution hepatitis C virus subtyping using NS5B deep sequencing and phylogeny, an alternative to current methods. J Clin Microbiol. 2015;53:219–226. doi: 10.1128/JCM.02093-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Guidelines for the screening, care and treatment of persons with hepatitis C infection. WHO Library Cataloguing-in-Publication 2014. Available from: http://www.who.int/hepatitis/hepatitis-c-guidelines/ [PubMed] [Google Scholar]
- 104.American Association for the Study of Liver Diseases (AASLD) and Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. Luxembourg: Publications Office of the European Union; 2014. Available from: http://www.hcvguidelines.org/full-report-view. [Google Scholar]
- 105.European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392–420. doi: 10.1016/j.jhep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 106.Kohli A, Shaffer A, Sherman A, Kottilil S. Treatment of hepatitis C: a systematic review. JAMA. 2014;312:631–640. doi: 10.1001/jama.2014.7085. [DOI] [PubMed] [Google Scholar]
- 107.Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, Schiff E, Ghalib R, Ryan M, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–1888. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 108.Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz E, Everson G, Bennett M, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 109.Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, Svarovskaia E, Brainard DM, Symonds WT, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370:1993–2001. doi: 10.1056/NEJMoa1316145. [DOI] [PubMed] [Google Scholar]
- 110.Ferenci P. Treatment of hepatitis C in difficult-to-treat patients. Nat Rev Gastroenterol Hepatol. 2015;12:284–292. doi: 10.1038/nrgastro.2015.53. [DOI] [PubMed] [Google Scholar]
- 111.Koff RS. Review article: the efficacy and safety of sofosbuvir, a novel, oral nucleotide NS5B polymerase inhibitor, in the treatment of chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2014;39:478–487. doi: 10.1111/apt.12601. [DOI] [PubMed] [Google Scholar]
- 112.Lawitz E, Poordad F, Brainard DM, Hyland RH, An D, Dvory-Sobol H, Symonds WT, McHutchison JG, Membreno FE. Sofosbuvir with peginterferon-ribavirin for 12 weeks in previously treated patients with hepatitis C genotype 2 or 3 and cirrhosis. Hepatology. 2015;61:769–775. doi: 10.1002/hep.27567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Akkarathamrongsin S, Payungporn S, Thong VD, Poovorawan K, Prapunwattana P, Poovorawan Y, Tangkijvanich P. Early viral kinetics during hepatitis C virus genotype 6 treatment according to IL28B polymorphisms. World J Gastroenterol. 2014;20:10599–10605. doi: 10.3748/wjg.v20.i30.10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sullivan JC, De Meyer S, Bartels DJ, Dierynck I, Zhang EZ, Spanks J, Tigges AM, Ghys A, Dorrian J, Adda N, et al. Evolution of treatment-emergent resistant variants in telaprevir phase 3 clinical trials. Clin Infect Dis. 2013;57:221–229. doi: 10.1093/cid/cit226. [DOI] [PubMed] [Google Scholar]
- 115.Ogert RA, Howe JA, Vierling JM, Kwo PY, Lawitz EJ, McCone J, Schiff ER, Pound D, Davis MN, Gordon SC, et al. Resistance-associated amino acid variants associated with boceprevir plus pegylated interferon-α2b and ribavirin in patients with chronic hepatitis C in the SPRINT-1 trial. Antivir Ther. 2013;18:387–397. doi: 10.3851/IMP2549. [DOI] [PubMed] [Google Scholar]
- 116.Barnard RJ, Howe JA, Ogert RA, Zeuzem S, Poordad F, Gordon SC, Ralston R, Tong X, Sniukiene V, Strizki J, et al. Analysis of boceprevir resistance associated amino acid variants (RAVs) in two phase 3 boceprevir clinical studies. Virology. 2013;444:329–336. doi: 10.1016/j.virol.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 117.Poveda E, Wyles DL, Mena A, Pedreira JD, Castro-Iglesias A, Cachay E. Update on hepatitis C virus resistance to direct-acting antiviral agents. Antiviral Res. 2014;108:181–191. doi: 10.1016/j.antiviral.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 118.Jacobson IM, Dore GJ, Foster GR, Fried MW, Radu M, Rafalsky VV, Moroz L, Craxi A, Peeters M, Lenz O, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403–413. doi: 10.1016/S0140-6736(14)60494-3. [DOI] [PubMed] [Google Scholar]
- 119.Vidal LL, Santos AF, Soares MA. Worldwide distribution of the NS3 gene 80K polymorphism among circulating hepatitis C genotype 1 viruses: implication for simeprevir usage. J Antimicrob Chemother. 2015;70:2024–2027. doi: 10.1093/jac/dkv081. [DOI] [PubMed] [Google Scholar]
- 120.Nakamoto S, Kanda T, Wu S, Shirasawa H, Yokosuka O. Hepatitis C virus NS5A inhibitors and drug resistance mutations. World J Gastroenterol. 2014;20:2902–2912. doi: 10.3748/wjg.v20.i11.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McPhee F, Hernandez D, Yu F, Ueland J, Monikowski A, Carifa A, Falk P, Wang C, Fridell R, Eley T, et al. Resistance analysis of hepatitis C virus genotype 1 prior treatment null responders receiving daclatasvir and asunaprevir. Hepatology. 2013;58:902–911. doi: 10.1002/hep.26388. [DOI] [PubMed] [Google Scholar]
- 122.Grebely J, Dore GJ. Can hepatitis C virus infection be eradicated in people who inject drugs? Antiviral Res. 2014;104:62–72. doi: 10.1016/j.antiviral.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 123.McGowan C, Harris M, Rhodes T. Hepatitis C avoidance in injection drug users: a typology of possible protective practices. PLoS One. 2013;8:e77038. doi: 10.1371/journal.pone.0077038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Reimer J, Schmidt CS, Schulte B, Gansefort D, Gölz J, Gerken G, Scherbaum N, Verthein U, Backmund M. Psychoeducation improves hepatitis C virus treatment during opioid substitution therapy: a controlled, prospective multicenter trial. Clin Infect Dis. 2013;57 Suppl 2:S97–104. doi: 10.1093/cid/cit307. [DOI] [PubMed] [Google Scholar]
- 125.Korthuis PT, Feaster DJ, Gomez ZL, Das M, Tross S, Wiest K, Douaihy A, Mandler RN, Sorensen JL, Colfax G, et al. Injection behaviors among injection drug users in treatment: the role of hepatitis C awareness. Addict Behav. 2012;37:552–555. doi: 10.1016/j.addbeh.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Backmund M, Meyer K, Edlin BR. Infrequent reinfection after successful treatment for hepatitis C virus infection in injection drug users. Clin Infect Dis. 2004;39:1540–1543. doi: 10.1086/425361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Grebely J, Knight E, Ngai T, Genoway KA, Raffa JD, Storms M, Gallagher L, Krajden M, Dore GJ, Duncan F, et al. Reinfection with hepatitis C virus following sustained virological response in injection drug users. J Gastroenterol Hepatol. 2010;25:1281–1284. doi: 10.1111/j.1440-1746.2010.06238.x. [DOI] [PubMed] [Google Scholar]
- 128.Grebely J, Pham ST, Matthews GV, Petoumenos K, Bull RA, Yeung B, Rawlinson W, Kaldor J, Lloyd A, Hellard M, et al. Hepatitis C virus reinfection and superinfection among treated and untreated participants with recent infection. Hepatology. 2012;55:1058–1069. doi: 10.1002/hep.24754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hagan LM, Yang Z, Ehteshami M, Schinazi RF. All-oral, interferon-free treatment for chronic hepatitis C: cost-effectiveness analyses. J Viral Hepat. 2013;20:847–857. doi: 10.1111/jvh.12111. [DOI] [PubMed] [Google Scholar]
- 130.Zanini B, Benini F, Pigozzi MG, Furba P, Giacò E, Cinquegrana A, Fasoli M, Lanzini A. Addicts with chronic hepatitis C: difficult to reach, manage or treat? World J Gastroenterol. 2013;19:8011–8019. doi: 10.3748/wjg.v19.i44.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tovo CV, de Mattos AA, de Almeida PR. Chronic hepatitis C genotype 1 virus: who should wait for treatment? World J Gastroenterol. 2014;20:2867–2875. doi: 10.3748/wjg.v20.i11.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Calvo-Cidoncha E, González-Bueno J, Almeida-González CV, Morillo-Verdugo R. Influence of treatment complexity on adherence and incidence of blips in HIV/HCV coinfected patients. J Manag Care Spec Pharm. 2015;21:153–157. doi: 10.18553/jmcp.2015.21.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Razavi H, Waked I, Sarrazin C, Myers RP, Idilman R, Calinas F, Vogel W, Mendes Correa MC, Hézode C, Lázaro P, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21 Suppl 1:34–59. doi: 10.1111/jvh.12248. [DOI] [PubMed] [Google Scholar]
- 134.Aspinall EJ, Corson S, Doyle JS, Grebely J, Hutchinson SJ, Dore GJ, Goldberg DJ, Hellard ME. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis. 2013;57 Suppl 2:S80–S89. doi: 10.1093/cid/cit306. [DOI] [PubMed] [Google Scholar]


