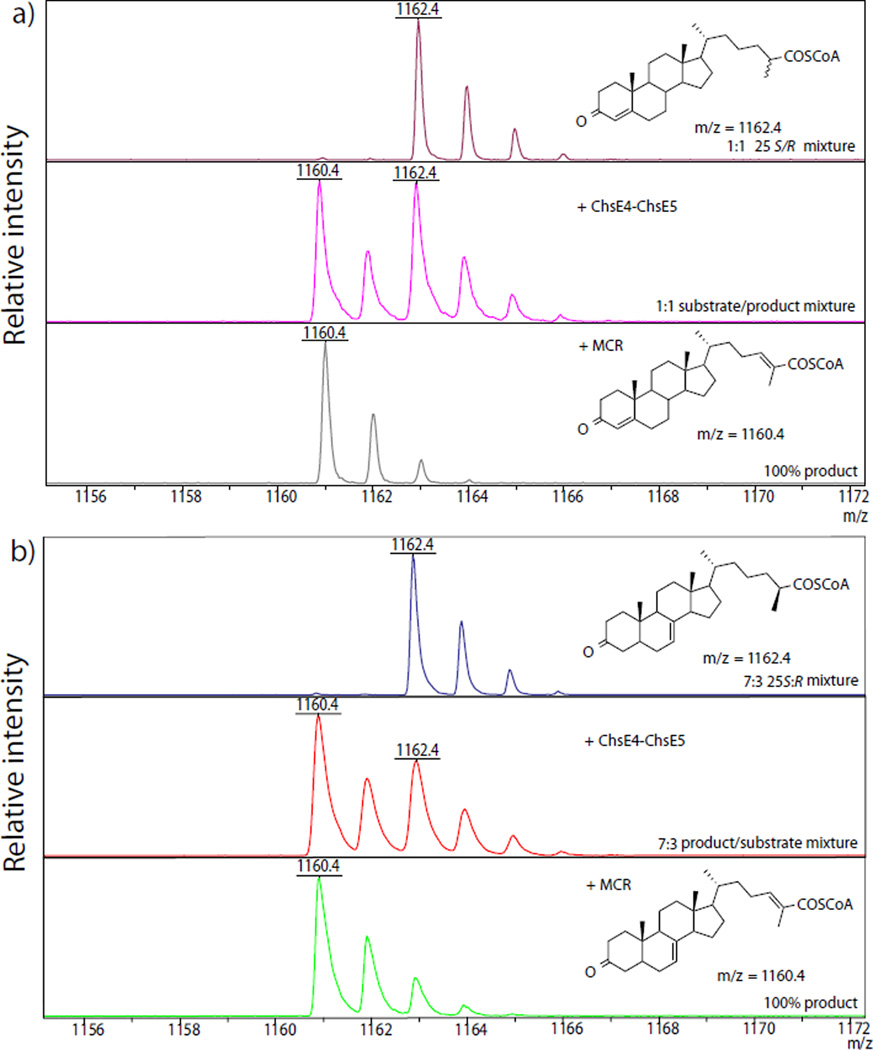
Figure 1.
ChsE4-ChsE5 and MCR product analysis by MALDI-TOF mass spectrometry illustrating that ChsE4-ChsE5 is stereospecific for the 25S steroyl-CoA diastereomer. a) 1:1 (25R:25S)-3-OCS-CoA substrate (top), product of ChsE4-ChsE5 catalyzed dehydrogenation (middle), product after addition of MCR to the ChsE4-ChsE5 reaction mixture (bottom). b) 7:3 (25S:25R)-Δ7-dafachronyl-CoA substrate (top), product of ChsE4-ChsE5 catalyzed dehydrogenation (middle), product after addition of MCR to the ChsE4-ChsE5 reaction mixture (bottom). In each case, reaction mixtures were monitored until no further changes in product distribution occurred.