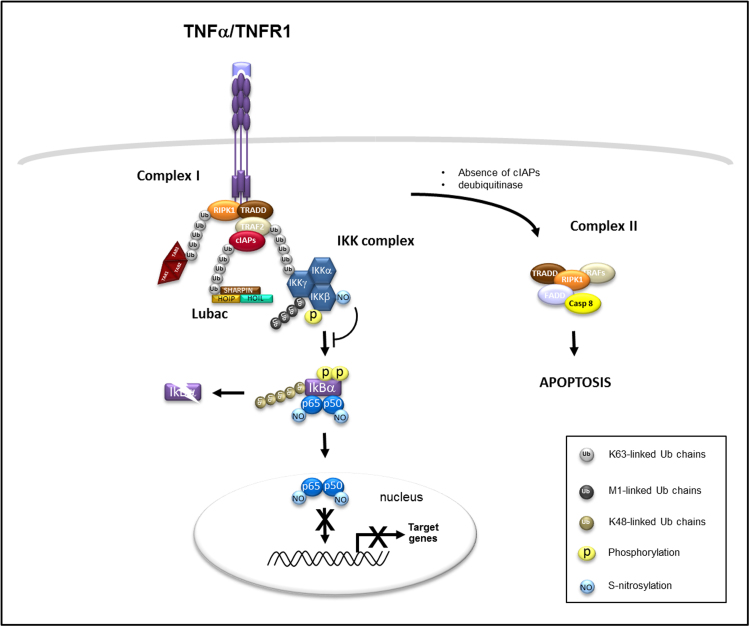
Fig. 2.
S-nitrosylation in classical TNF-NF-kB signaling. When TNFα binds its receptor TNF receptor 1 (TNFR1), it stimulates the formation of complex I that results in the recruitment of TNFR-associated via death domain (TRADD), receptor-interacting serine/threonine protein kinase 1 (RIPK1), TNFR-associated factor 2 (TRAF2), cellular inhibitor of apoptosis cIAPs (cIAP1 and cIAP2). The cIAPs, via their E3 ubiquitin ligases activities, promote the ubiquitination (through K63-linked ubiquitin chains) of RIPK1, TRAF2, and themselves which subsequently allows the recruitment of other complexes. Linear ubiquitin chain assembly complex (LUBAC) associates with ubiquitin linkages attached to cIAPs, transforming growth factor-β activated kinase (TAK1)/TAK1-binding 2 (TAB2)/TAK1 binding 3 (TAB3) associates with ubiquitin linkages attached to RIPK1 and inhibitor of kappaB alpha (IkBα) kinase (IKK) complex associates with ubiquitin linkages attached to TRAF2. LUBAC ubiquitylates (through M1-linked ubiquitin chains) IKKγ and stably recruits the IKK complex. The IKK complex (IKKβ) is then phosphorylated by TAK1/TAB2/TAB3. IKKβ in turn phosphorylates IkBα that leads to the addition of K48-linked ubiquitin chains and its proteasome-mediated degradation with the subsequent activation and nuclear translocation of the NF-kB transcription factor heterodimer. S-nitrosylation of IKKβ subunit prevents the phosphorylation of IkBα and its degradation. S-nitrosylation of either p50–p65 NF-kB subunits inhibits DNA binding and transcriptional upregulation of NF-kB target genes. In the absence of cIAPs or in presence of CYLD, which allows the removal of K63-linked ubiqtuitin chains, RIPK1 translocate to the cytoplasm to induce the formation and activation of complex II that leads to apoptosis.