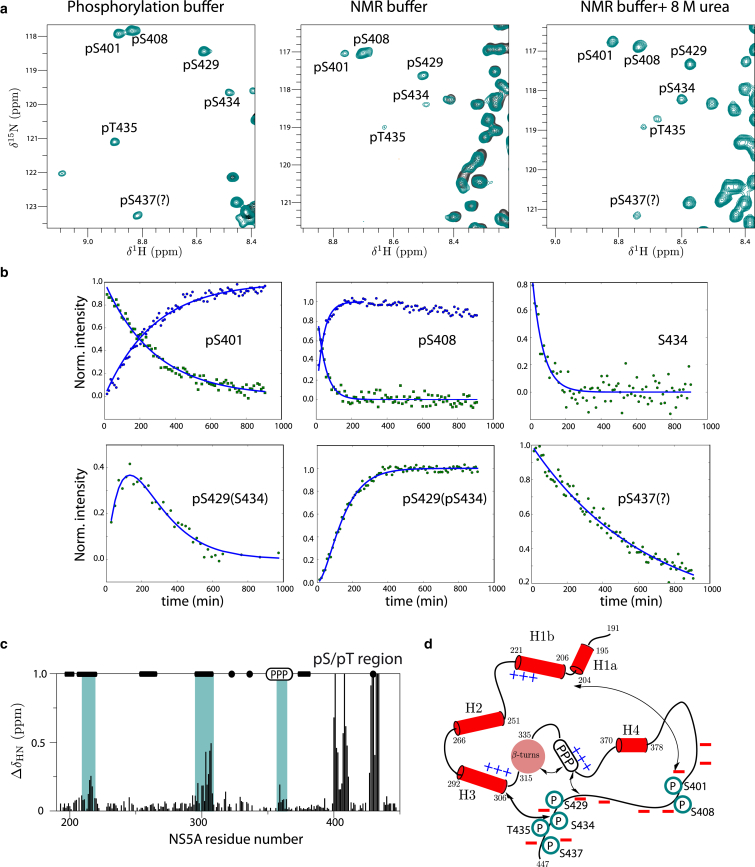
Figure 7.
Phosphorylation study of NS5A-D2D3 by CK2. (a) 1H-15N correlation spectra of NS5A-D2D3 (green) measured 380 min after adding CK2 kinase. The spectral region, displaying cross peaks of phosphoserine and phosphothreonine residues (annotated in the spectra), is shown for the sample in the phosphorylation buffer, in the NMR buffer, and after adding 8 M urea. (Black) Reference spectrum before phosphorylation. (b) Kinetics traces (normalized peak intensity plotted as a function of reaction time) are shown for representative cross peaks of residues directly involved in phosphorylation, or close to a phosphorylation site. The kinetic data were fitted either to a model assuming a single phosphorylation event (and therefore single-exponential kinetics), or (in the case of intermediates, i.e., intensity buildup followed by decay) to a model of two consecutive phosphorylation events. (Lines) Results of the fitting. (c) 1H-15N chemical shift changes in NS5A-D2D3 upon phosphorylation by CK2 indicating structural changes in remote regions (indicated by green bars). (d) Model of the long-range electrostatic interactions with negative charges introduced by the phosphorylation of residues in D3. To see this figure in color, go online.