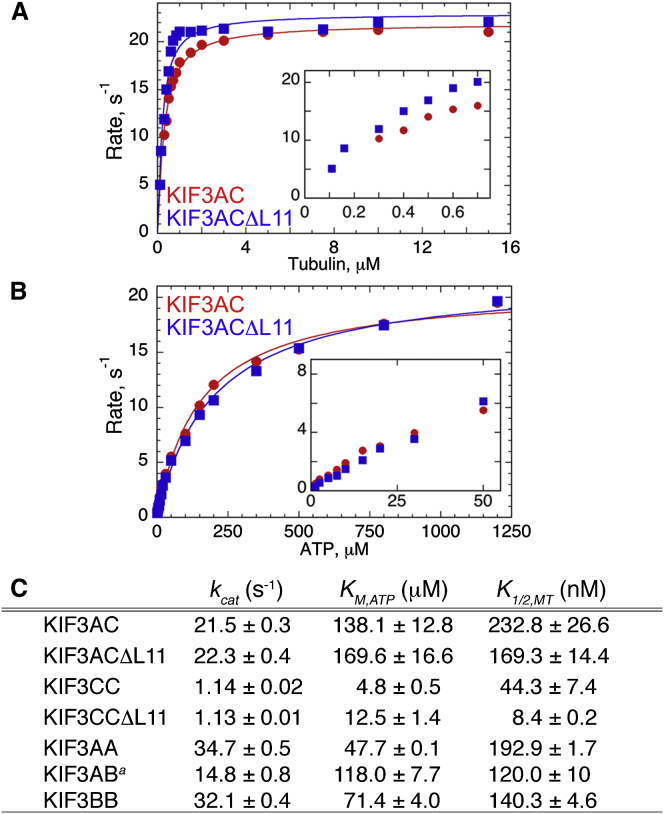
Figure 6.
Shortening loop L11 increases apparent microtubule affinity. (A–C) Steady-state ATPase kinetics of KIF3AC and KIF3ACΔL11 as a function of microtubule (A) or MgATP (B) concentration. (A) Final concentrations: 0.1 μM KIF3AC or KIF3ACΔL11 active sites, 2 mM Mg[α-32P]ATP, 40 μM paclitaxel, and 0.1–15 μM tubulin polymer. The quadratic fit provided the kcat and K1/2,MT for each motor. (Inset) Low concentrations of tubulin polymer (0–0.8 μM) illustrate the difference in apparent microtubule affinity of KIF3AC vs. KIF3ACΔL11. (B) Final concentrations: 0.1 μM KIF3AC or KIF3ACΔL11 active sites, 20 μM tubulin polymer, 40 μM paclitaxel, and 1.5–1200 μM Mg[α-32P]ATP. The Michaelis-Menten fit provided the kcat and KM,ATP for each motor. (C) Table of the steady-state ATPase parameters reported as mean ± SE.