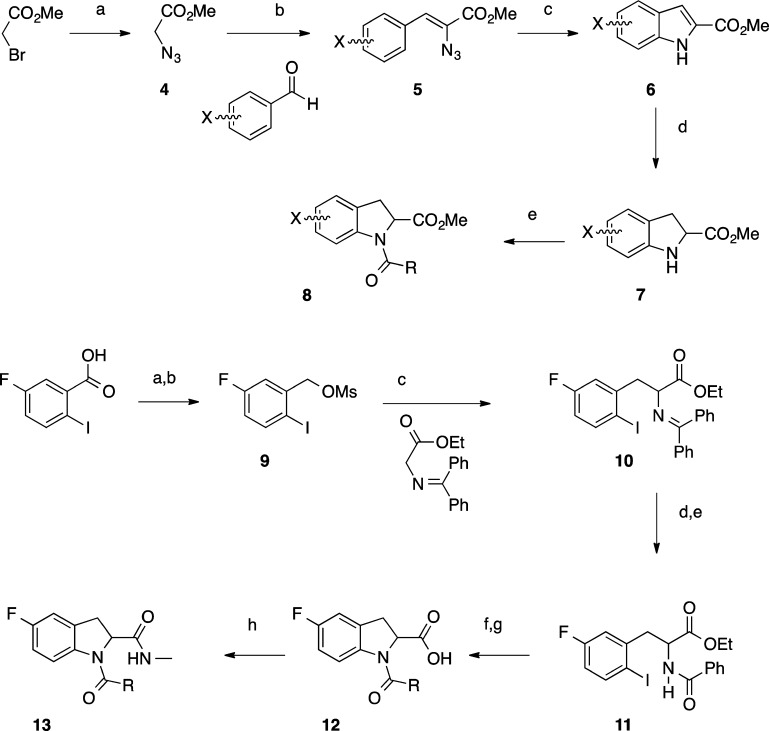
Scheme 2.
Conditions (4–8): (a) NaN3, DMF, 0 °C to room temperature, 16 h, under Ar; (b) methyl azidoacetate (3 mol equiv), aldehyde (1 mol equiv), THF, NaOMe, MeOH, −10 °C, 3 h; (c) Rh2(O2CC3F7)4, toluene, 65 °C, 48 h; (d) Mg, MeOH, 0 °C to room temperature, 16 h, under Ar; (e) MeNH2 in THF, room temperature, 16 h, then RCOCl, DIPEA, DCM, room temperature under argon. Conditions (9–13): (a) BH3 1 M in THF 0 °C to room temperature, 16 h; (b) MsCl, DIPEA, THF, 0 °C, 2 h; (c) TBAI, 5 mol % in toluene, 50% (w/v) NaOH, 0 °C to room temperature, 16 h; (d) citric acid, 0 °C to room temperature, 3 h; (e) RCO2H, TBTU, DIPEA, room temperature, 2 h; (f) CsOAc, CuI, DMSO, room temperature, 1.5 h; (g) LiOH, THF, room temperature; (h) TBTU, MeNH2 (2 M in MeOH), DCM, room temperature, 2 h. Overall 46% yield from commercially available 5-fluoro-2-iodobenzoic acid.