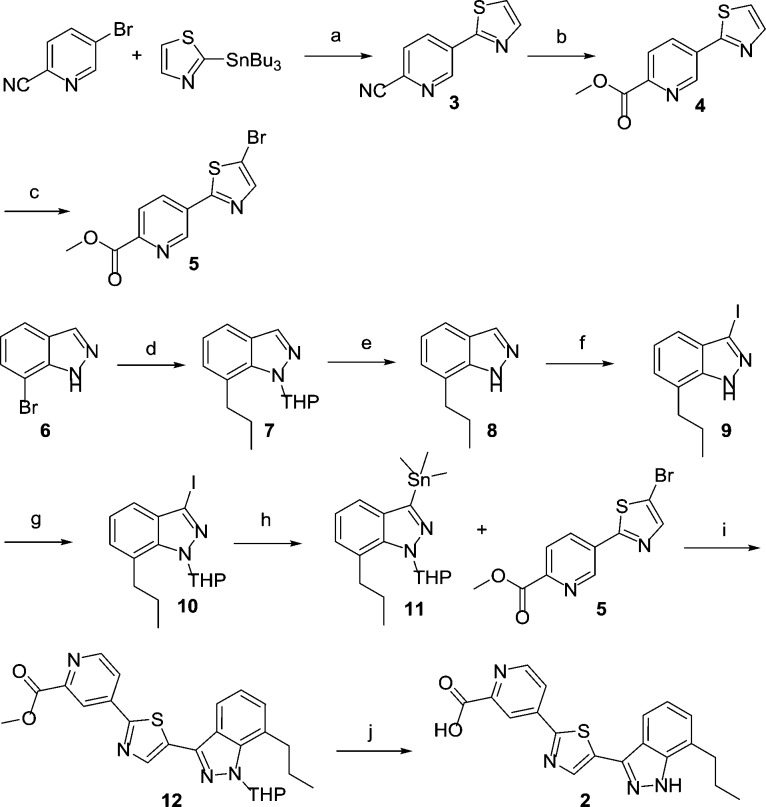
Scheme 1. Synthesis of Indazoles.
Reagents and conditions: (a) Pd(PPh3)2Cl2, 1,4-dioxane, 110 °C,82%; (b) 4 M HCl in dioxane, MeOH, 75 °C, 87%; (c) NBS, acetonitrile, 90 °C, 78%; (d) 1. dihydropyran, TFA, 90 °C; 2. n-PrB(OH)2, Pd(dppf)Cl2, aq. Na2CO3, 1,4-dioxane, 110 °C, 53%; (e) TFA, DCM, RT, 87%; (f) KOH, I2, RT, 75%; (g) dihydropyran, TFA, 90 °C, 65%; (h) Pd(PPh3)2Cl2, Me6Sn2, 1,4-dioxane, 100 °C, 65%; (i) Pd(PPh3)2Cl2, P(furyl)3, 1,4-dioxane, 100 °C, 52%; (j) 6 M aq HCl, 100 °C, 76%.