Figure 2.
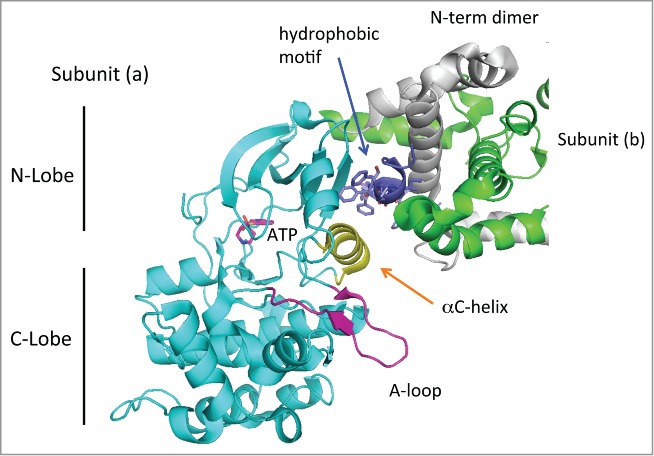
Structure of the dimeric MRCKβ catalytic domain. The dimeric MRCKβ is based on the coordinates in 3TKU. The catalytic domain reveals a typical structure, with the disposition of C- and N-lobe as indicated. The activation loop (purple) is fully ordered, and the aC-helix (yellow) lies in a position that would be compatible with catalysis. The position of ATP as indicated is based on binding of Fasudil in this crystal structure.40 The hydrophobic motif (dark blue) binds to the N-lobe as is typical for other kinases, but this region makes substantial contacts also with the N-terminal helices that are involved in dimerization. Thus the dimerization domain may be required for holding the hydrophobic motif.
