Figure 3.
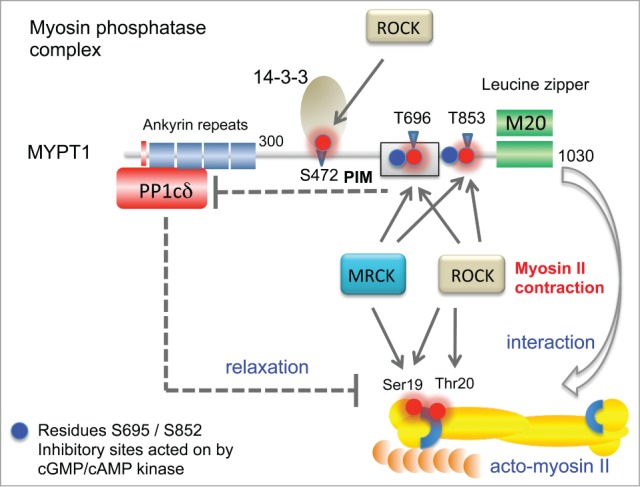
Features of the MYPT1 (PPP1R12A) phosphatase complex and its activity toward myosin regulatory light chain (MLC2). The catalytic PP1δ subunit binds MYPT1 at an RVxF motif found at amino acids 35–38 (red bar). Ankyrin repeats (x8) are further involved to bind the protein phosphatase catalytic subunit PP1c. When the complex is in the vicinity of the myosin complex, it can act on MLC2. MYPT1 can be phosphorylated at Thr696 (PIM, phosphatase inhibitory motif) by both ROCK and MRCK, which allows this region to inhibit the PP1c (dotted line). Although MYPT1 Thr853 phosphorylation is also inhibitory, the mechanism is not so well understood. Other important regulatory sites marked as blue spheres are regulated by cGMP- or cAMP-dependent kinases 71; these help maintain MYPT1 activity by preventing ROCK phosphorylation of neighboring residues. Phosphorylation of Ser472 generates a site that allows for recruitment of 14–3–3, which prevents myosin interaction. The third subunit of the MYPT1 holoenzyme is termed M20 which binds to and indeed resembles MYPT1 residues 934–1006. This region of the complex is thought to allow targeting the bipolar myosin heavy chain complexes as indicated (arrow).
