Figure 1.
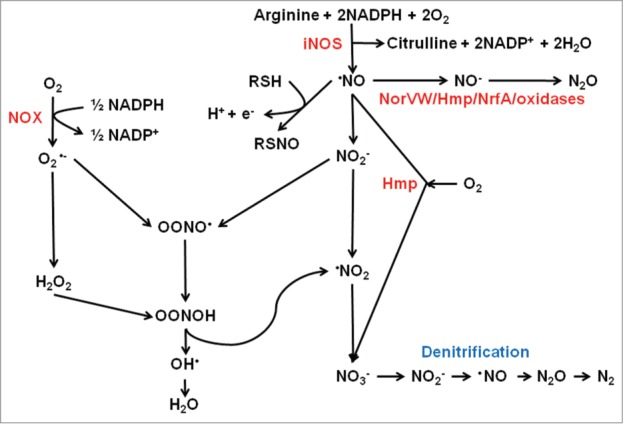
Interplay between reactive oxygen and reactive nitrogen species. The host enzyme NADPH oxidase (NOX) generates superoxide (O2•−) from O2. Aerobic metabolism within the pathogen inevitably results in side reactions in which successive one electron reductions of O2 yields the reactive oxygen species, O2•−, hydrogen peroxide (H2O2) and the hydroxyl radical (•OH). Nitric oxide (NO) is generated by the action of host inducible nitric oxide synthase (iNOS) (and by some bacteria that possess nitric oxide synthase). Nitric oxide is a reactive free radical and is a source of reactive nitrogen species such as nitroxyl (NO−), nitrosonium (NO+), and peroxynitrite (ONOO−), which is formed by reaction of NO with O2•−, or NO− and O2, and peroxynitrous acid (OONOH). Nitric oxide reacts with thiol groups to modify activity by the formation of S-nitrosylated proteins (RSNO). Nitric oxide can be detoxified by the flavohemoglobin Hmp by conversion to nitrate (NO3−) in the presence of O2. Some bacteria are capable of denitrification in which NO3− is converted to nitrogen gas (N2) via NO as an intermediate. In the absence of O2, the major detoxification mechanism in E. coli is the anaerobic NO reductase NorVW (O2-sensitive flavorubredoxin) that converts 2NO to N2O (nitrous oxide) and water. A similar reaction can be catalyzed by Hmp and NrfA in the absence of O2. Some terminal oxidases can also reduce NO to N2O, or by reaction of ferryl heme (Fe4+ = O2−) with NO generate NO2−.
