Figure 4.
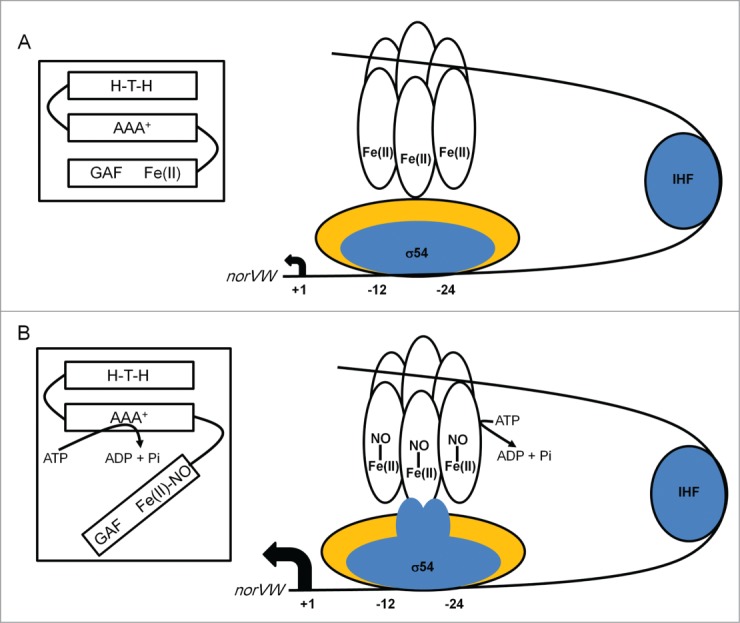
Scheme summarizing the action of NorR at the E. coli norVW promoter. (A) In the absence of NO hexameric NorR (unfilled ovals) is able to bind to enhancer elements located upstream of the norVW core σ54-dependent promoter elements (-12 and -24) via its helix-turn-helix (H-T-H) DNA-binding domain. Integration host factor (IHF) bends the DNA such that NorR and the σ54-RNA polymerase holoenzyme can potentially interact. However, these interactions are unproductive because the ATPase activity of the NorR AAA+ domain is inhibited by interaction with the GAF domain, which contains the sensory mononuclear iron center (Fe[II]) (see inset). Consequently, norVW transcription is switched off (small filled arrow, +1). (B) When NO binds at the mononuclear iron centers of NorR (Fe[II]-NO) the AAA+ domain is released from the sensory GAF domain (see inset) and acquires ATPase activity allowing productive interactions with σ54-RNA polymerase. The ensuing conformational changes promote the formation of the open complex and enhance norVW transcription (large filled arrow, +1). For clarity, not all the regulatory elements operating at this promoter are shown. The diagram is not drawn to scale.
