Figure 5.
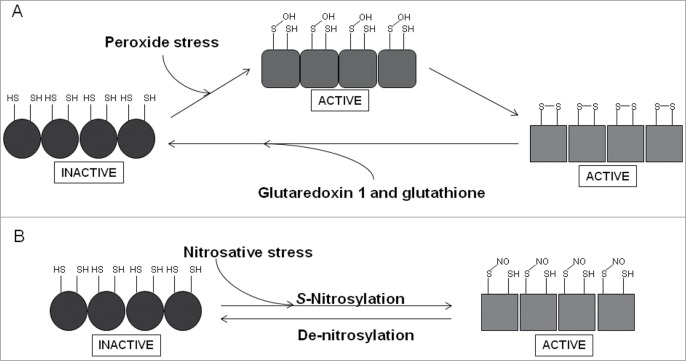
Scheme summarizing the redox-reactivity of OxyR. (A) The sensory C-terminal domain of each monomer in the OxyR homotetramer contains a redox-reactive cysteine residue (Cys-199), which forms a sulfenic acid (S-OH) in the presence of peroxide stress. This form of OxyR is proposed to be able to regulate gene expression, although more likely acts as an intermediate in forming the true active form, which is able to bind DNA and serve as a transcriptional regulator and contains an intra-molecular disulphide bond between Cys-199 and Cys-208. OxyR returns to its inactive form (Cys-199, SH; Cys-208, SH) by the action of glutaredoxin 1 and glutathione. (B) A secondary role of OxyR is as a nitrosative stress responder. S-nitrosylation of Cys-199, forming S-NO, leads to activation of OxyR, de-nitrosylation, forming SH, returns OxyR to its inactive form.
