Figure 1.
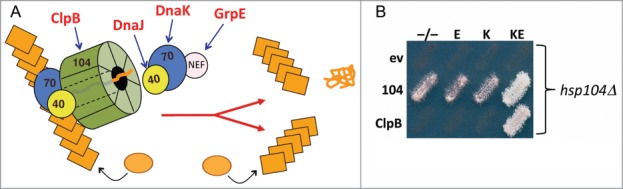
E. coli disaggregation machinery propagates prions in yeast. (A) Yeast prions propagate as amyloid fibers (stacked orange rectangles) that grow when soluble protein (orange circles) is added to fiber ends. Replication of fibers requires cooperation of the Hsp70 system (Hsp70, Hsp40 and NEF) with Hsp104 (green), which extracts proteins from the fiber thereby causing the fiber to break into 2 self-assembling prion fibers. E. coli counterparts of machinery components are indicated in red. (B) Cells lacking chromosomal Hsp104 (hsp104Δ) express Hsp104 (104) or E. coli ClpB from a plasmid, or carry the corresponding empty vector (ev), as indicated on the left. As indicated above, cells also carry plasmids for expressing E. coli Hsp70 DnaK (K), the DnaK NEF GrpE (E), both (KE) or the empty vectors (−/−). Medium shown allows growth only if cells propagate prions. The combination of ClpB, DnaK and GrpE (BKE) is both necessary and sufficient for prion propagation when Hsp104 is absent.
