Figure 2.
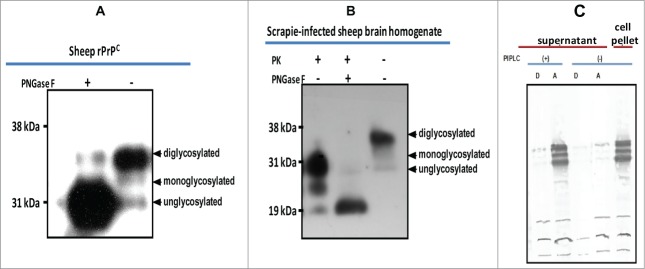
Characterization of baculovirus-expressed PrPC. (A) Cleavage of N-linked glycans from rPrPC by PNGaseF treatment. (B) Cleavage of N-linked glycans from sheep PrP derived from scrapie-infected sheep brain. A molecular weight shift confirms N-linked glycosylation of rPrPC characteristic of mammalian expressed PrPC. (C) GPI anchor assay using PIPLC and Triton X-114 phase partitioning. Cleavage by the enzyme releases the recombinant protein into the medium (supernatant), which upon partitioning was detected in the aqueous (A) phase. No recombinant protein could be detected in the supernatant of non-treated cells. The recombinant protein remained associated with the cell pellet in non-treated cells. Proteins were detected with the monoclonal antibody, mAbP4. PK = proteinase K.
