Figure 3.
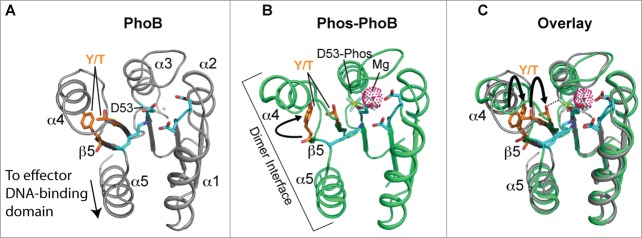
Structure and activation of REC domains. (A) Structure of the PhoB 2-component response regulator REC domain from Escherichia coli (PDB 1B00; ref. 158) shown in the inactivated, unphosphorylated state. PhoB exhibits a classical REC (α/β)5 topology, and α4-β5-α5 canonical dimeric interface. The proposed alternative dimer interface is the α1-α5.63,169 The tyrosine and threonine involved in ‘Y/T’ coupling are colored in orange. Notice the tyrosine is protruding into the solvent area of the α4-β5-α5 face to presumably prevent dimerization and activation. Key residues involved in the active pocket phosphorylation events are colored cyan, including the invariant aspartate residue (D53) that is phosphorylated by the cognate histidine kinase. Nitrogen atoms are colored blue and oxygen atoms red. (B) Structure of activated, phosphorylated PhoB (Phospho-PhoB; PDB 1ZES; ref. 62). Upon D53 phosphorylation, the conserved Y/T residues move toward the catalytic pocket (the Y movement is denoted by the arrow) to coordinate the new phosphate group (colored yellow) and allow the dimer to form. The magnesium ion and surrounding electron cloud (Mg) is shown in magenta. (C) Overlay of PhoB and Phospho-PhoB structures from A and B. The black arrows indicate the movements of the Y/T residues to allow dimerization about the α4-β5-α5 interface.
