Figure 9.
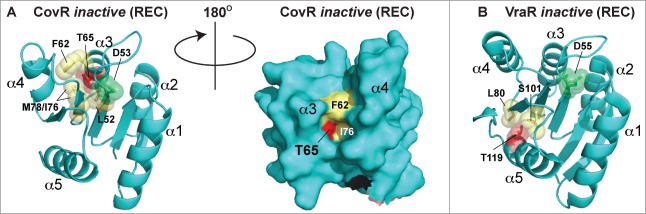
Comparison of CovR and VraR Receiver (REC) domain phosphorylation sites. (A) (left panel) Cartoon representation of an atomic model of the CovR REC domain. The model was created with Swiss-Model (ref.161) using the CovR sequence from S. agalactiae and the PhoP structure from M. tuberculosis (PDB code 3R0J; ref.165) as a molecular template. Phosphorylated residues are shown in red. The conserved histidine kinase phosphorylated aspartate (Asp53) is shown in green. Residues that would potentially pose a steric clash with the phosphorylated Thr65 are shown in yellow. (right panel) Surface representation of the left panel rotated 180 degrees. Notice that Thr65 is solvent accessible, but resides in a deep cleft within the interior of the protein. (B) The VraR atomic (REC) structure taken from ref.63 showing the eSTK-regulated Thr119 (colored red) and potential residues that might clash with it in its phosphorylated form (colored yellow). The conserved Asp55 is shown in green.
