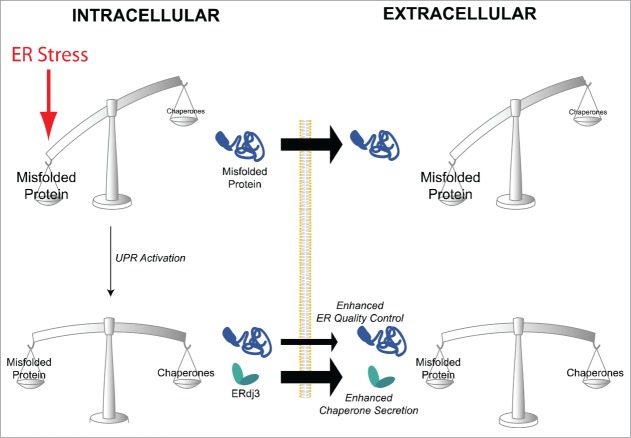
Figure 1.
ERdj3 secretion restores extracellular proteostasis during ER stress. In response to ER stress, the intracellular accumulation of misfolded proteins in the ER can facilitate the aberrant secretion of misfolded proteins to extracellular environments. The UPR-dependent increase in ERdj3 provides a mechanism to increase extracellular chaperoning capacity and attenuate the potentially proteotoxic accumulation and aggregation of these misfolded protein conformations in the extracellular space.