Figure 2.
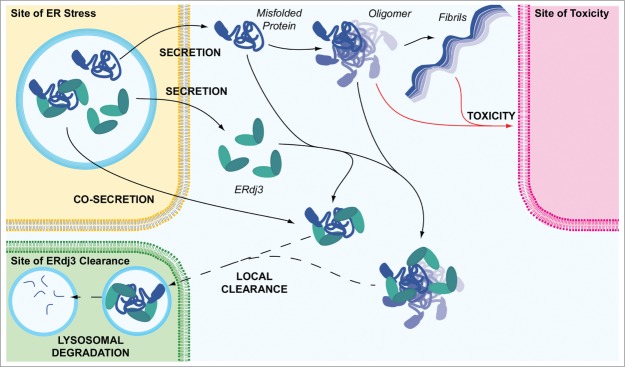
ERdj3 segregates proteotoxic protein conformations to protect the extracellular environment. ERdj3 can be secreted either as an unbound protein or bound to a destabilized protein. In the extracellular environment, unbound ERdj3 can complex with misfolded protein conformations or oligomers, preventing proteotoxic protein aggregation into soluble oligomers or amyloid fibrils that induce cellular toxicity. Based on comparisons with other secreted chaperones, ERdj3-substrate complexes are likely cleared from extracellular environments through a mechanism involving endocytosis and subsequent lysosomal degradation. ER stress and consequently increased secretion of misfolded, aggregation-prone proteins could have a particular impact on the cell types near the site of ER stress. Thus, increased ERdj3 secretion through the UPR could represent a mechanism to protect these local extracellular environments from proteotoxic protein conformations. In this model, ERdj3 and ERdj3-substrate complexes could potentially be locally cleared to protect the environment most threatened by the secretion of misfolded protein conformations to the extracellular space.
