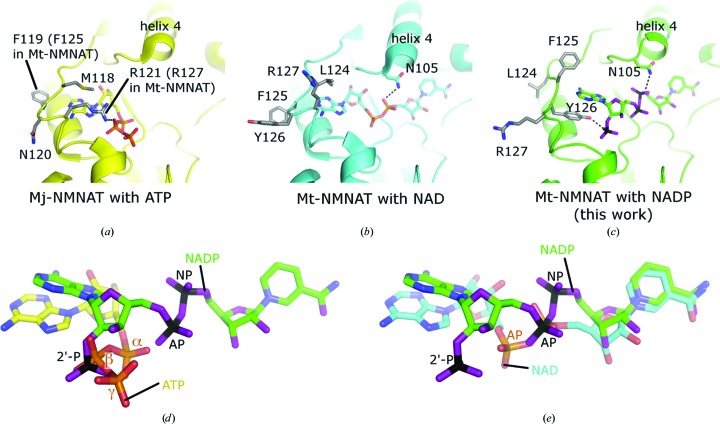
Figure 4.
Adjustments of the NMNAT-binding pocket to different nucleotides and alternative conformations. (a) NMNAT with the substrate ATP (PDB entry 1f9a; D’Angelo et al., 2000 ▸). (b) NMNAT with the reaction product NAD+ (PDB entry 1ej2; Saridakis et al., 2001 ▸). (c) NMNAT with NADP+ (PDB entry 4h6l; this work). (d) Position of NADP+ compared with that of ATP (PDB entry 1f9a). (e) Position of NADP+ compared with that of NAD+ (PDB entry 1ej2). (d) and (e) were obtained by superposing the entire NMNAT protein structures onto each other while omitting the ligands. In NADP+ the nucleotide is flipped by about 180° compared with NAD+ and ATP, thereby positioning the 2′-phosphate group at the position of the β-phosphate site of ATP. The positions of the central phosphate groups in NADP+ (AP and NP) have also slightly shifted compared with NAD+, whereas the position of the nicotinamide group is almost unchanged.