Figure 1.
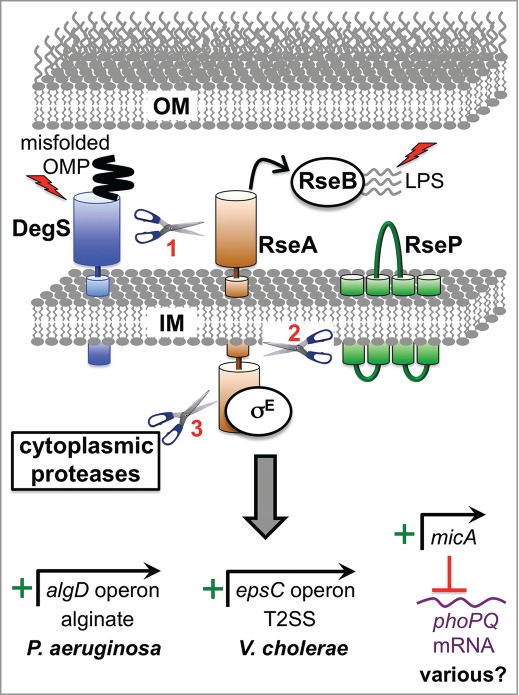
Examples of direct virulence gene regulation by the RpoE ESR. Two inducing signals (shown as lightening bolts) are required for activation. One is binding of a misfolded outer membrane protein (OMP) to DegS (AlgW in P. aeruginosa), which causes a conformational change that induces its proteolytic activity. The other is off-pathway LPS molecules, which bind to RseB (MucB in P. aeruginosa) and dissociate it from the antisigma factor RseA (MucA in P. aeruginosa). Both signals are required in order for DegS to cleave the periplasmic domain of RseA. The truncated RseA is then cleaved by the IM protease RseP (MucP in P. aeruginosa). This releases the cytoplasmic domain of RseA in complex with RpoE (σE; AlgU/T in P. aeruginosa), which is degraded by cytoplasmic proteases to release RpoE. RpoE-containing RNA polymerase binds directly to the algD promoter to induce alginate biosynthesis in P. aeruginosa and to the epsC promoter to induce T2SS production in V. cholerae. In E. coli K-12 the micA promoter is RpoE-dependent and drives the production of a non-coding sRNA that inhibits phoP translation (RpoE also induces 2 other sRNAs, RybB and MicL, which are not shown; see text). The predicted base pairing between MicA sRNA and phoPQ mRNA is conserved in pathogens including Salmonella, Shigella and Enterobacter. Therefore, RpoE might downregulate the virulence-associated PhoPQ regulon in multiple species. Mucoid P. aeruginosa strains from the lungs of individuals with cystic fibrosis most often have mutations that destroy antisigma factor RseA/MucA function, which leads to constitutive activation of RpoE, high algD operon expression and the production of large amounts of the exopolysaccharide, alginate.
