Figure 6.
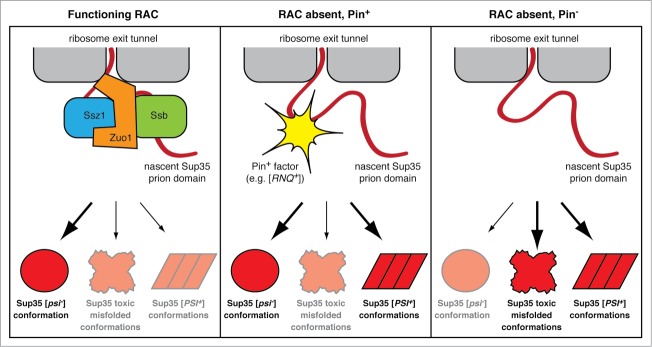
A model for the function of the RAC in antagonizing prion formation. (Left panel) RAC-mediated Ssb chaperoning of nascent Sup35 minimizes its co-translational conversion into non-native conformations, including toxic misfolded species and [PSI+] prion conformations. (Center panel) In the absence of RAC function, the N-terminal prion domain of Sup35 is more vulnerable to misfolding. [PIN+] factors, such as the [RNQ+] prion, can serve as templates to cross-seed Sup35 folding into [PSI+] prion conformations and thereby reduce the propensity of Sup35 to adopt more toxic conformations. (Right panel) In the absence of RAC function and without [PIN+] factors, Sup35 is free to misfold into a wide spectrum of conformations, including prion forms and toxic misfolded forms.
