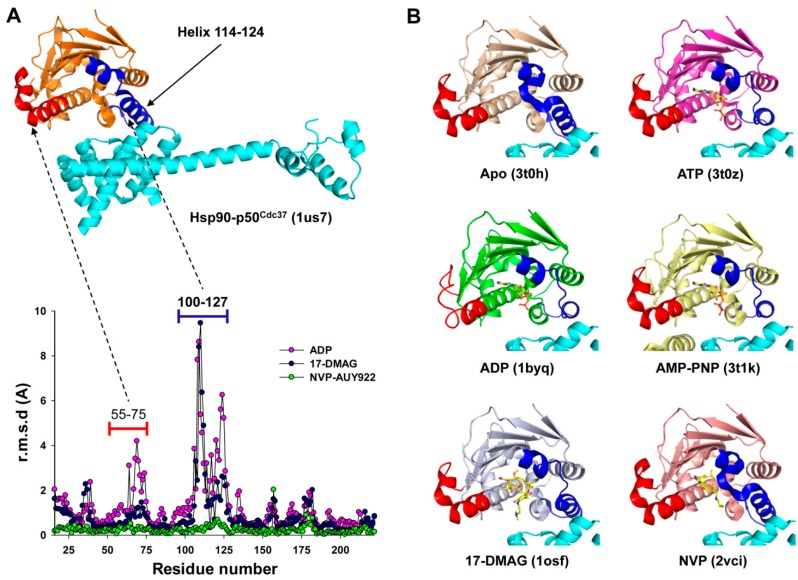
Figure 6.
ADP-induced structural changes in Hsp90 may impact complex formation with p50Cdc37. (A) Root mean square deviation (r.m.s.d.) analysis of the N-terminal domain of human Hsp90α in the ligand-free state (3T0H) against the liganded states with ADP (1BYQ), 17-DMAG (1OSF), or NVP-AUY922 (2VCI). Residues 100-127 and, to a lesser extent, residues 55-75, undergo large structural changes upon ligand binding, corresponding to the blue and red highlighted regions of the yeast Hsp90:human p50Cdc37 complex (1US7) shown at the top of the graph. p50Cdc37 is shown in cyan; (B) Superposition of different liganded states of the N-terminal domain of Hsp90 show conformational changes that may impact the interaction with p50Cdc37.