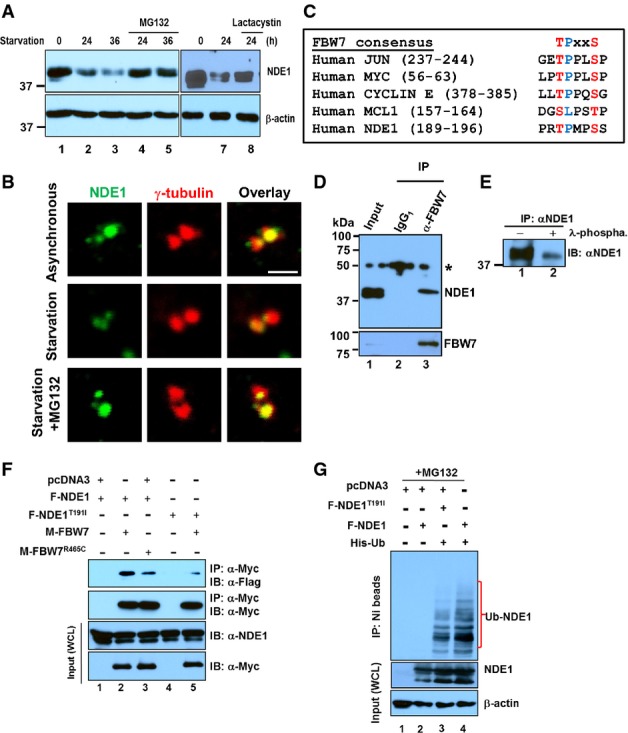
A Expression levels of NDE1 (upper panels) or β-actin (lower panels) in exponentially growing RPE1-hTERT cells (lanes 1 and 6) or serum-starved cells for the indicated time points (lanes 2–5 and 7 and 8). Cells were treated with proteasomal inhibitors, MG132 (10 μM, lanes 4 and 5) or lactacystin (5 μM, lane 8), for 4 h before the indicated collection time points.
B Expression levels of NDE1 at the centrosome in exponentially growing (asynchronous), 24-h serum starvation or 24-h serum-starved RPE1-hTERT cells treated for 4 h prior to fixation with MG132 (10 μM). Cells were double stained with rabbit α-NDE1 (green) and mouse γ-tubulin (red). Scale bar: 1 μm.
C FBW7 phosphodegron sequences in human JUN, MYC, CYCLIN E, MCL1, and NDE1.
D Physical interaction of endogenous FBW7 and NDE1 in RPE1-hTERT cells. Control mouse IgG1 (lane 2) or mouse α-FBW7 (lane 3) was added to cell lysates, and captured immunocomplexes (lanes 2 and 3) were immunoblotted with rabbit α-NDE1 (upper panel). Expression of NDE1 (upper panel) or FBW7 (lower panel) in lysates is shown in lane 1. Immunoprecipitated FBW7 is shown in the lower panel (lane 3). Asterisk indicates non-specific band.
E NDE1 is endogenously phosphorylated in hRPE1-hTERT cells. Immunoprecipitated NDE1 was left untreated (lane 1) or treated with λ-phosphatase.
F F-Box pathogenic mutation R456C in FBW7 or T191I within the FBW7 recognition site in NDE1 suppresses interaction between FBW7 and NDE1. HEK293T cells were transfected with indicated plasmids and serum starved for 24 h before lysis. Cells were treated with MG132 (10 μM) for 4 h prior to cell lysis. Wild-type and mutant form of Myc-tagged FBW7 was immunoprecipitated with α-Myc, and complexes were blotted with α-Flag to detect wild-type or mutant Flag-tagged NDE1. Expression levels of Myc- or Flag-tagged proteins in whole cell lysates (WCL) are shown in the lower panels.
G NDE1 is ubiquitylated in vivo. HEK293T cells were co-transfected with Flag-tagged wild-type F-NDE1 or NDE1T191I (F-NDE1T91I) and His-tagged ubiquitin (His-Ub). Cells were lysed in a denaturing buffer, and His-Ub was purified over a nickel column (NI beads). Protein complexes covalently bound to His-Ub were immunoblotted with α-NDE1 (upper panel). Expression levels of wild-type and mutant NDE1 or β-actin are shown in middle or lower panel, respectively. Cells were treated with 10 μM MG132 for 4 h before lysis.