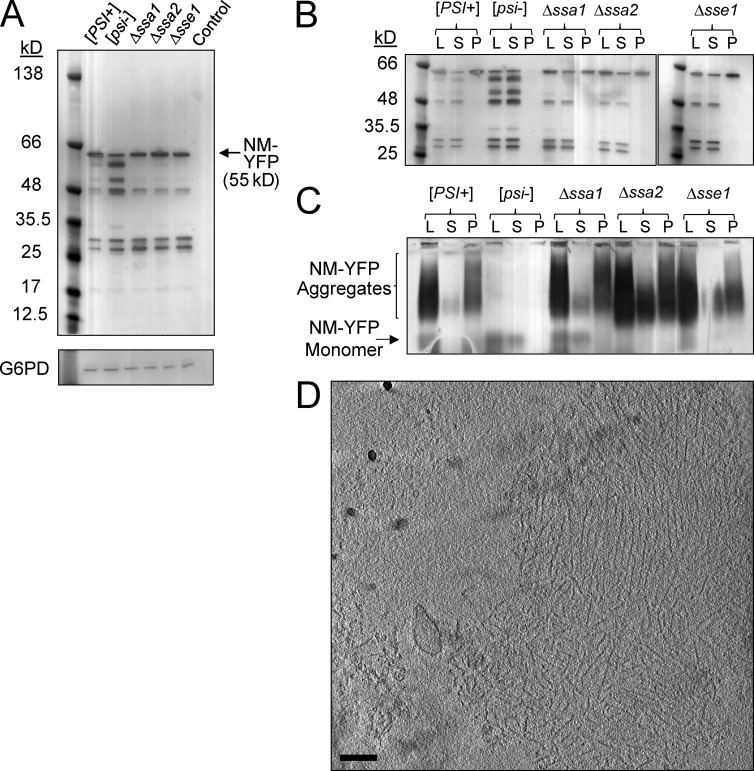
Figure 2.
Chaperone deletion alters the size distribution of NM-YFP aggregates. (A) Western blot of cell lysates using an α-YFP antibody showing NM-YFP expression levels in [PSI+], [psi−], Δssa1, Δssa2, and Δsse1 cells. The parental wild-type strain, devoid of the NM-YFP expression cassette, but otherwise isogenic, is included as a control. An α-Glucose-6-phosphate dehydrogenase (G6PD) antibody was used to verify equal loading of the lysates. (B) Ultracentrifugation sedimentation of NM-YFP aggregates in lysates of [PSI+], [psi−], Δssa1, Δssa2, and Δsse1 cells, analyzed by Western blot using an α-YFP antibody. Lysate (L), supernatant (S), and pellet (P) fractions are indicated. (C) SDD-AGE analysis and immunoblotting of the ultracentrifugation fractions described in B. (D) Slice through a representative cryotomogram of an NM-YFP dot aggregate isolated from the pellet fraction of the ultracentrifuged [PSI+] cell lysate. Of seven individual pelleted dots observed, 43% had preserved the fibril array architecture, as shown, whereas the remainder consisted of disordered clusters of NM-YFP fibrils. The disorder in fibril packing for these dots had been introduced by sample handling, because all dot assemblies from five independent dot aggregates observed directly in the lysates, without ultracentrifugation, had a well-preserved fibril array arrangement consistent with the example shown. Bar, 200 nm.