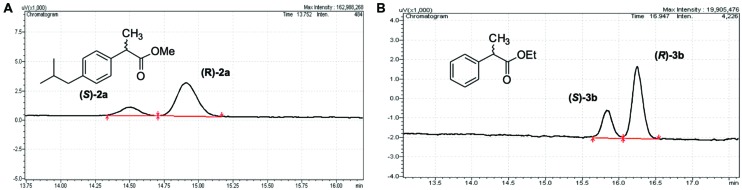
FIGURE 4.
Enantioselectivity of PL-GOS. PL-GOS preferably hydrolyzes the R-enantiomers of Ibuprofen methyl ester (A) and phenylpropionic acid methyl ester (B). Racemic mixtures of the respective esters were incubated with PL-GOS, then products were derivatized with TMS-diazomethane and separated on a chiral gas chromatography column.