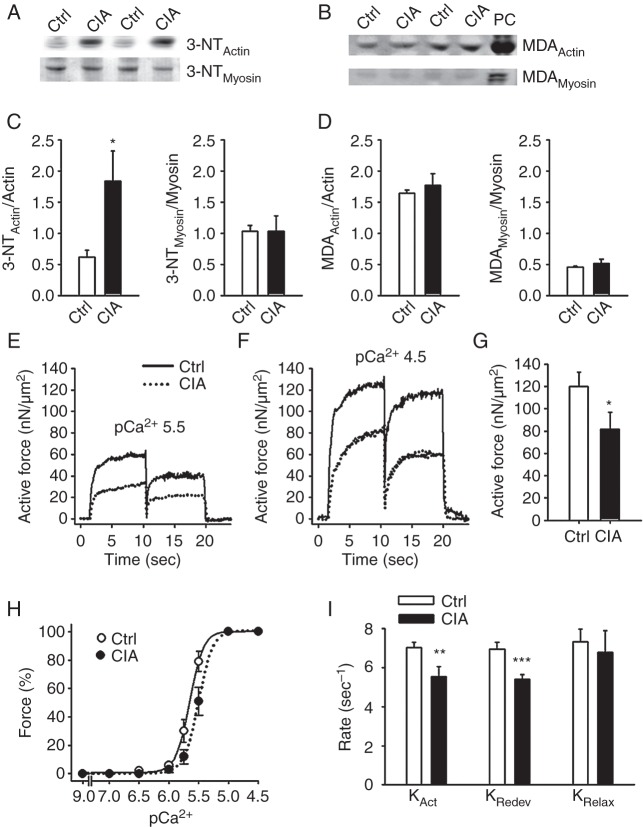
Figure 5.
Nitration of actin and decreased actin–myosin interaction underlie weakness in CIA muscles. Western blots assessing 3-NT (A) and MDA (B) accumulation on actin and myosin in fast-twitch muscles from CIA and control mice. Hydrogen peroxide (200 µM, 20 min) was used as positive control (PC). Mean data of 3-NT (C) and MDA (D) accumulation on actin and myosin normalised to total protein (n=6). (E, F) Typical records of active force from atomic force cantilever measurements in myofibrils in pCa2+ of 5.5 and 4.5, respectively. (G) Mean values of the active isometric forces produced by myofibrils at a sarcomere length of 2.5 µm and pCa2+ 4.5 (n=12). (H) Mean values of the rates of force development during initial activation (KAct), after shortening (KRedev), and the fast rate of relaxation following deactivation (KRelax) (n=6). (I) Myofibril force-pCa2+ relation, generated by plotting the mean force against the pCa2+ at a sarcomere length 2.5 µm. There was a rightward shift in the curve from CIA myofibrils (pCa2+50(Ctrl): 5.65 vs pCa2+50(CIA): 5.5; p<0.05), whereas the steepness was not significantly affected (NCtrl: 4.87 vs NCIA: 4.56). Data are mean±SEM; *p<0.05; **p<0.01; ***p<0.001 versus controls.