Abstract
Autoimmune myositis encompasses various myositis-overlap syndromes, each being identified by the presence of serum marker autoantibodies. We describe a novel myositis-overlap syndrome in 4 patients characterized by the presence of a unique immunologic marker, autoantibodies to nuclear pore complexes. The clinical phenotype was characterized by prominent myositis in association with erosive, anti-CCP, and rheumatoid factor-positive arthritis, trigeminal neuralgia, mild interstitial lung disease, Raynaud phenomenon, and weight loss. The myositis was typically chronic, relapsing, and refractory to corticosteroids alone, but remitted with the addition of a second immunomodulating drug. There was no clinical or laboratory evidence for liver disease. The prognosis was good with 100% long-term survival (mean follow-up 19.5 yr).
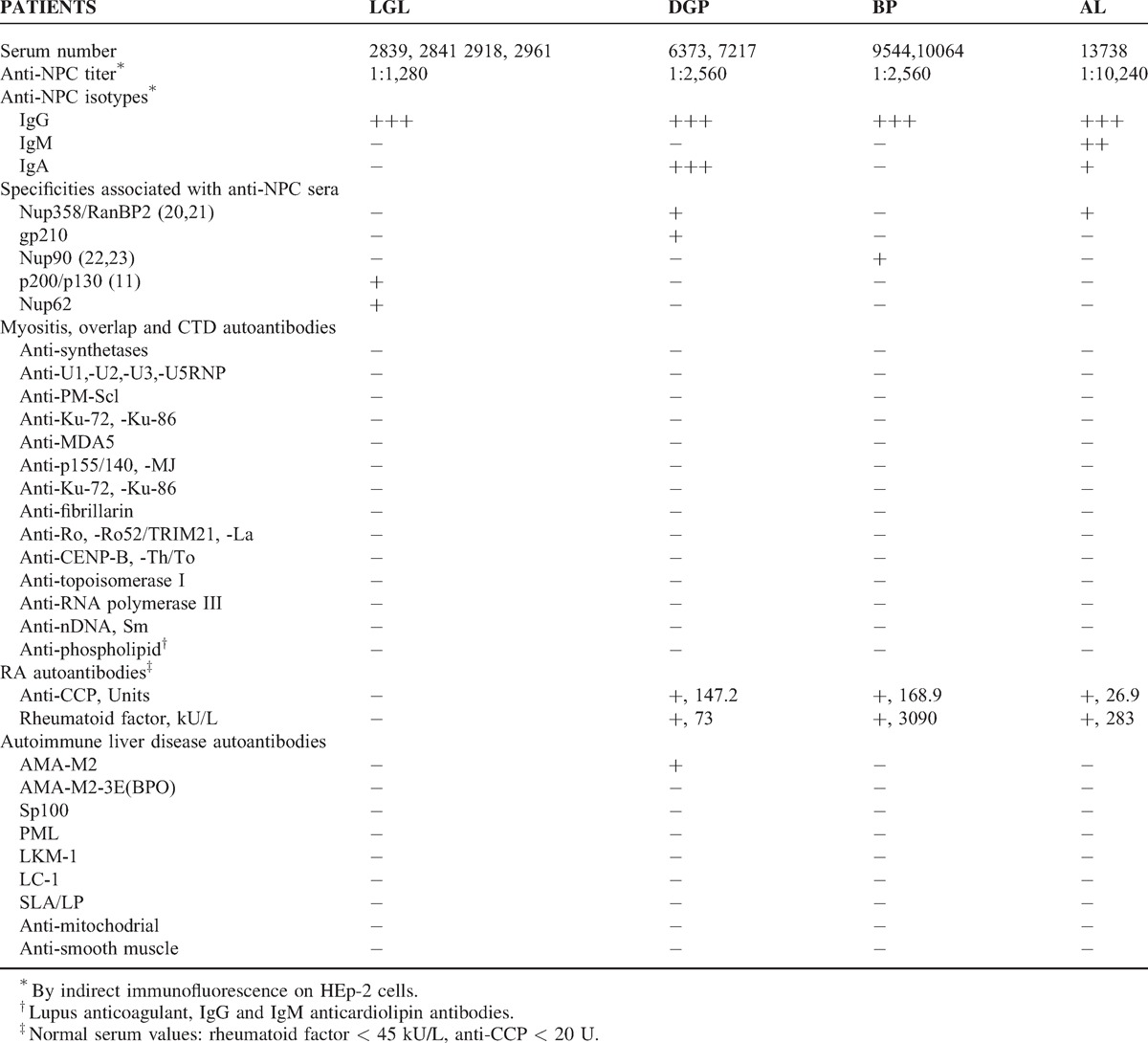
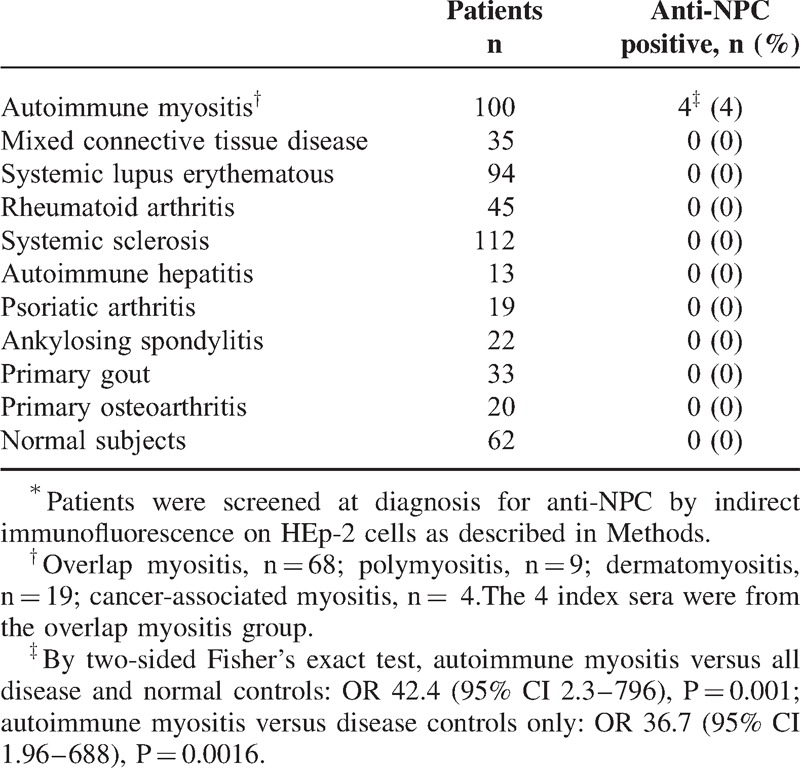
By indirect immunofluorescence on HEp-2 cells, sera from all 4 patients displayed a high titer of antinuclear autoantibodies (ANA) with a distinct punctate peripheral (rim) fluorescent pattern of the nuclear envelope characteristic of nuclear pore complexes. Reactivity with nuclear pore complexes was confirmed by immunoelectron microscopy. In a cohort of 100 French Canadian patients with autoimmune myositis, the nuclear pore complex fluorescent ANA pattern was restricted to these 4 patients (4%). It was not observed in sera from 393 adult patients with systemic sclerosis (n = 112), mixed connective tissue disease (n = 35), systemic lupus (n = 94), rheumatoid arthritis (n = 45), or other rheumatic diseases (n = 107), nor was it observed in 62 normal adults.
Autoantibodies to nuclear pore complexes were predominantly of IgG isotype. No other IgG autoantibody markers for defined connective tissue diseases or overlap syndromes were present, indicating a selective and highly focused immune response. In 3 patients, anti-nuclear pore complex autoantibody titers varied in parallel with myositis activity, suggesting a pathogenic link to pathophysiology. The nuclear pore complex proteins, that is, nucleoporins (nup), recognized by these sera were heterogeneous and included Nup358/RanBP2 (n = 2 patients), Nup90 (n = 1), Nup62 (n = 1), and gp210 (n = 1). Taken together the data suggest that nup autoantigens themselves drive the anti-nup autoimmune response. Immunogenetically, the 4 patients shared the DQA1∗0501 allele associated with an increased risk for autoimmune myositis.
In conclusion, we report an apparent novel subset of autoimmune myositis in our population of French Canadian patients with connective tissue diseases. This syndrome is recognized by the presence of a unique immunologic marker, autoantibodies to nuclear pore complexes that react with nups, consistent with an “anti-nup syndrome.”
INTRODUCTION
Autoantibodies to nuclear antigens (ANAs) are important diagnostic and prognostic markers in the autoimmune connective tissue diseases.23,50,52 ANAs are routinely detected by indirect immunofluorescence on cultured human cells such as HEp-2 cells. Fluorescent patterns as detected on ANA substrates provide clues to the antigenic specificity of ANAs and to the presumptive molecular identity of cognate autoantigens. Typical examples are the speckled fluorescent ANA pattern associated with anti-U1RNP in mixed connective tissue disease (MCTD) and the centromere fluorescent ANA pattern, which suggests the presence of anti-CENP-B autoantibodies associated with the limited cutaneous subset of systemic sclerosis.23,50,52
Nuclear envelope acquisition is a landmark event in the evolution of eukaryotic cells. By encasing the genome, this distinct double lipid membrane forms a physical barrier facing externally the cytoplasm and the endoplasmic reticulum lumen and, internally, the nuclear chromatin.18,44 The transport of macromolecules between the nucleus and the cytoplasm is mediated through nuclear pore complexes (NPCs), which generate a highly selective and dynamic permeability barrier.14,18a,20 Each NPC has a core structure consisting of a hollow cylinder embedded in the nuclear envelope and is composed of about 30 different proteins termed nucleoporins (nups).14,18a,20,28 The NPC structure contains 3 major domains: the central framework consisting of the selective central channel, or central transporter region, the cytoplasmic ring composed of a protein complex and cytoplasmic filaments, and the nuclear ring with nucleus extending filaments forming a basket-like structure. The different nups are distributed in 1 of these 3 NPC domains. Nups can be divided in 2 main classes. The first class of nups represents a stable scaffold involved in stabilization and maintenance of the pore membrane, whereas the second class includes peripheral and mobile nups responsible for transport functions. In addition to their transport roles, nups have critical roles in chromatin organization, mitosis, DNA repair and regulation of gene expression.14,18a,20,28 Given the critical roles of the NPC in cellular functions, it is not surprising that a growing number of reports reveal the involvement of nups in specific human diseases.6,44
In 1988, our team described an autoantibody to NPCs (anti-NPC) in serum from patient LGL with autoimmune myositis (AIM) and trigeminal neuralgia.10 This autoantibody was associated with a characteristic punctate peripheral (rim) fluorescent nuclear pattern on HEp-2 cells and was shown by immunogold electron microscopy to react with NPCs on the cytoplasmic side of the nuclear envelope.10 Anti-NPC persisted in serial serum samples over 2 years and appeared to fluctuate in parallel with myositis activity.
Subsequently, a small number of anti-NPC autoantibodies were found to be associated with organ-specific autoimmune diseases.11 As an example, autoantibodies to the nups gp210 and p62 were associated with primary biliary cirrhosis (PBC), occurring in 25% of patients.11,55 However, no study has reported thus far on the frequency of anti-NPC in a large population of patients with connective tissue diseases.
Since the original description of anti-NPC in patient LGL, we have identified in a cohort of French Canadian patients with connective tissue diseases 3 additional patients with a characteristic anti-NPC fluorescent ANA pattern. As in the case of patient LGL, these patients were referred for evaluation of a connective tissue disease. We present herein the clinical and serologic course of these 4 patients over prolonged follow-up. These patients share a peculiar overlap connective tissue syndrome and clinical phenotype characterized by prominent myositis. This syndrome is associated with a good overall prognosis and prolonged survival. Immunologically, anti-NPC were of IgG isotype that targeted several nup autoantigens and were not associated with other major autoantibody markers. Moreover, anti-NPC titers fluctuated in parallel with myositis activity, suggesting a relationship to pathogenesis.
PATIENTS AND METHODS
Patients and Controls
The 4 index patients with anti-NPC were from a cohort of adult French Canadian patients with connective tissue diseases evaluated and followed longitudinally between 1984 and 2001 by our team at the Connective Tissue Diseases Clinic, Division of Rheumatology, Centre Hospitalier de l’Université de Montréal (CHUM), as described.24,37,41,50 Patients recruited in this cohort are classified as systemic sclerosis or systemic lupus erythematosus (SLE) according to American College of Rheumatology (ACR) disease criteria. Patients with AIM were classified as overlap myositis, polymyositis, dermatomyositis and cancer-associated myositis, as described.50 Definitions for monophasic versus chronic myositis, myositis relapse and refractory myositis were as reported by Troyanov et al.50 Control sera were from normal adults, and from patients with rheumatoid arthritis (defined according to ACR criteria), MCTD,52 autoimmune hepatitis, psoriatic arthritis, ankylosing spondylitis, primary gout or primary osteoarthritis, as described.41 Patients provided informed written consent for clinical data and serum collection. Biobank and clinical databank procedures were approved by the CHUM Ethical Review Board.
Identification of Anti-NPC Autoantibodies
In all index and control patients, serum samples obtained at the time of diagnosis and at follow-up were coded and studied blindly by 1 of us (JLS) at the screening dilution of 1:40 for the presence of ANAs by indirect immunofluorescence on HEp-2 cells (Antibodies Inc., Davis, CA), as described.38–40 The presence of autoantibodies to NPCs was identified by the characteristic punctate peripheral (rim) nuclear fluorescent pattern.10,11 Anti-NPC pattern on HEp-2 cells is readily distinguished from the linear continuous peripheral ANA pattern associated with anti-lamin B1 autoantibodies, and from the broad peripheral pattern associated with anti-DNA antibodies.40 Specific NPC localization of cognate autoantigens recognized by anti-NPC was further demonstrated by immunogold electron microscopy on nuclear envelopes and reported elsewhere.9,27,40,53,54
Variation of anti-NPC over time was studied by indirect immunofluorescence on coded serum samples obtained at follow-up visits and biobanked at −80°C. Fluorescence intensity of the NPC pattern on a scale of 0 to 4 at the screening serum dilution (1:40) on HEp-2 cells was rated blindly by a single observer, in single experiments for each patient. The fluorescence intensity at the screening dilution was used as a surrogate for end-point titers. This was correlated with serum creatine kinase levels (CK) measured on simultaneous serum samples, as an indicator of muscle necrosis.4
Isotypic analysis for IgG, IgM, and IgA anti-NPC was performed by indirect immunofluorescence on HEp-2 cells (Antibodies Inc.) using FITC-conjugated anti-human γ, μ and α heavy chain-specific antibodies (Cappel Laboratories, Cochranville, PA).
Line Immunoassay (LIA) and Addressable Laser Bead Immunoassay (ALBIA)
These methods were performed by 1 of us (MJF) as described.22 Autoantibodies against Jo-1, PL-7, PL-12, Ku-72, Ku-86, PM-Scl, and Mi-2 autoantigens were assayed by line immunoassay (LIA) (Euroline-WB assay, Euroimmun AG, Luebeck, Germany). Anti-fibrillarin reactivity was tested by ALBIA (Quanta-Plex9, INOVA Diagnostics, Inc., San Diego, CA) on a Luminex 100 flow fluorometer (Luminex Corp., Austin, TX) using purified recombinant fibrillarin protein (Mikrogen GmbH, Neuried, Germany).22
Anti-Nup62 reactivity was tested by addressable laser bead immunoassay (ALBIA) using recombinant human Nup62 (Diarect AG, Freiberg, Germany). Autoantibodies to the M2 mitochondrial autoantigens AMA-M2 and M2-3E(BPO), and to the autoantigens gp210, Sp 100, liver-kidney-microsome-1 (LKM-1), promyelocytic leukemia cell antigen (PML), liver cytosolic-1 antigen (LC-1), soluble liver antigen/liver pancreas antigen (SLA/LP), and Ro52/TRIM21 were tested by LIA (Euroimmun AG) as described.43
Protein A-Assisted Immunoprecipitation
Sera were analyzed by 1 of us (INT) for autoantibodies by protein A-assisted immunoprecipitation, both for nucleic acid analysis (RNA silver stain) and for proteins (metabolically labelled with 35S-methionine), along with double immunodiffusion.1,45,47,50 These immunoassays detect all of the described antisynthetases (Jo-1, PL-7, PL-12, OJ, EJ, KS, Tyr and Zo), anti-PM-Scl, anti-SumoAE, anti-RNA polymerase III, anti-Th/To, anti-U2RNP, anti-U3RNP, anti-U5RNP, anti-SRP, anti-Mi-2, anti-p155/140 and anti-MJ. Nucleic acid analysis used 3-5 mg of protein A-Sepharose, 20 μL of patient serum, and unlabeled HeLa cell extract (>106 cells). Immunoprecipitates were analyzed on 7 to 8 M urea, and 10% polyacrylamide gel electrophoresis with silver stain development. Protein analysis used 1 to 2 mg of protein A-Sepharose, 10–15 μL of serum, and 35S-methionine-labeled HeLa cell extract (>105 HeLa cells). Immunoprecipitates were analyzed by SDS-polyacrylamide gel electrophoresis (between 8% and 10%). None of the 4 index patient sera with anti-NPC had any of these autoantibodies. All 4 index sera were also negative by immunoprecipitation-blotting for anti-p155/140, anti-MJ, and anti-MDA5.48
Other Immunoassays
Anti-CCP antibodies were detected by ELISA (CCP3, INOVA Diagnostics).36 Values <20 units were considered negative, between 20 and 39 low positive, 40 to 59 moderate positive, and ≥60 high positive. Autoantibodies to Ro, La, Sm, U1RNP, topo, and Jo-1 as well as rheumatoid factor were determined by ELISA (Calbiotech, Spring Valley, CA). Anti-native DNA antibodies were measured by filter-binding assay.40 Anti-topoisomerase I antibodies were detected by ELISA using native full-length topoisomerase I extracted and purified from calf thymus (Immunovision, Springdale, AR).17 Anti-CENP-B was tested by ELISA using recombinant full-length CENP-B.51 Anti-mitochondrial antibodies (AMA) and anti-smooth muscle antibodies (SMA) were tested by indirect immunofluorescence on mouse tissues (CHUM Immunology Laboratory).
Capillary Microscopy
Semiquantitative nailfold capillary microscopy was performed at baseline and follow-up visits.24
HLA Genotyping
Genomic DNA was extracted from peripheral blood mononuclear cells using standard methods. HLA-DR, DQ, and DP alleles were determined by polymerase chain reaction and sequence specific oligonucleotide probes (PCR-SSOP) genotyping assay by 1 of us (RG, for DQA1 alleles)35 and HLA Tissue Typing Service, ProImmune Ltd, Oxford, United Kingdom.
RESULTS
Identification of 4 Patients With Autoantibodies to NPCs
As part of the routine evaluation of patients referred to the CHUM Connective Tissue Diseases Clinic, a serum sample is obtained, coded and studied blindly for ANAs by indirect immunofluorescence on HEp-2 cells.38–40 In sera from 4 patients (LGL, DGP, BP, and AL), the presence of anti-NPC autoantibodies was identified by the characteristic punctate peripheral (rim) nuclear fluorescent pattern,10,11 as shown in Figure 1. Immunogold electron microscopic studies published elsewhere using serum from patients LGL, DGP, and BP have confirmed specific NPC localization.9,10,27,53,54
FIGURE 1.
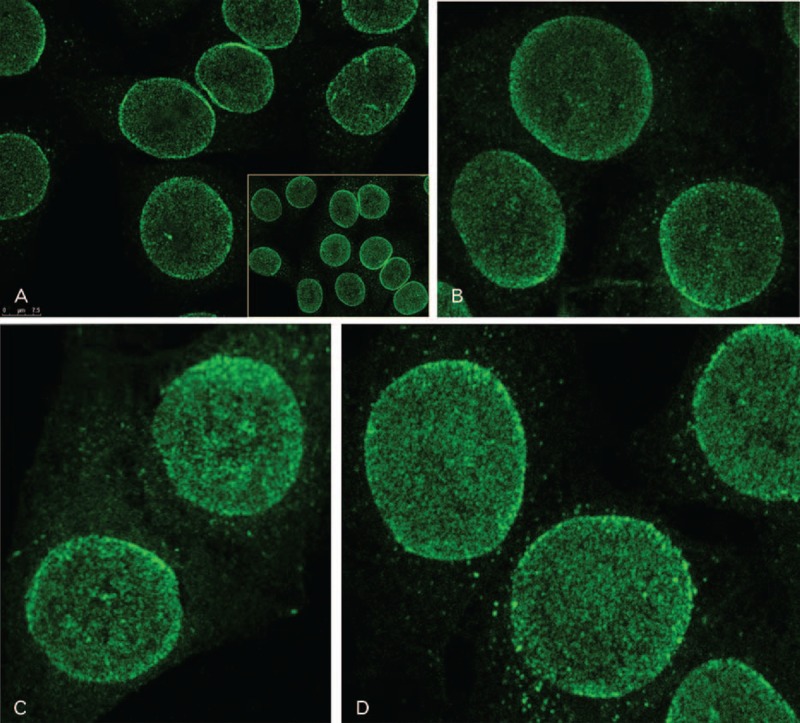
Autoantibodies to nuclear pore complexes are associated with a punctate peripheral (rim) ANA pattern. A. Indirect immunofluorescence on HEp-2 cells was performed using serum from patient AL at 1:40 dilution. Cells were observed by confocal microscopy. At lower magnification (inset), a dense punctate (speckled) nuclear staining is observed with a striking accentuation at the periphery of interphase nuclei. Confocal microscopy analysis revealed that the punctate staining was not present in the nuclear interior, and was restricted to the nuclear envelope. At higher magnification, the dense and finely speckled nuclear envelope staining is highlighted, as well as the punctate peripheral (rim) pattern. Discrete cytoplasmic speckles correspond to nuclear pore complexes within annulate lamellae, an endoplasmic reticulum subdomain containing densely packed pore complexes.20 Sera from the 3 other index patients revealed a similar pattern.10,27,53,54 B. Patient DGP; C, Patient BP; D, Patient LGL.
Anti-NPC Are Associated Clinically With Autoimmune Myositis
Having identified at referral the anti-NPC shared by these patients gave us the opportunity to characterize their clinical manifestations and long-term outcome. Table 1 shows these features before and at myositis diagnosis and over a mean follow-up of 19.5 years.
TABLE 1.
Clinical manifestations before and at myositis diagnosis and long-term clinical outcome in 4 patients with autoantibodies to nuclear pore complexes
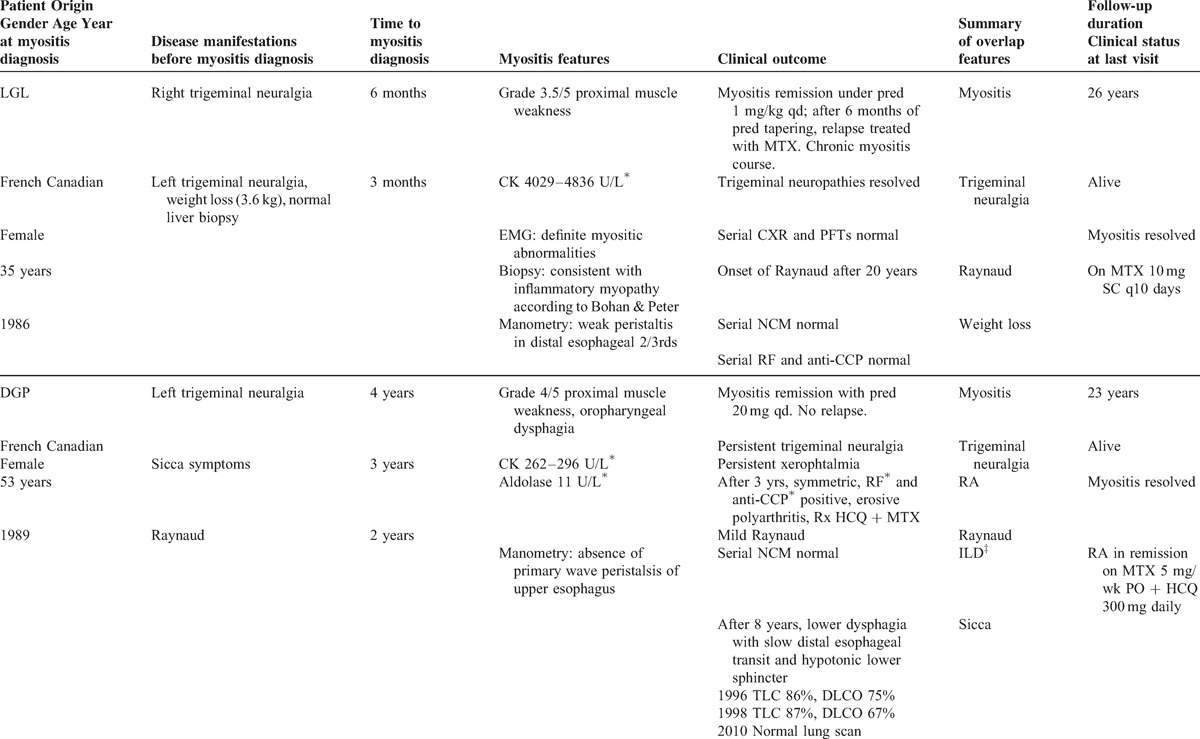
All 4 patients were French Canadian. There were 3 women and 1 man, with ages at the time of referral ranging from 35 years to 70 years. In the 4 patients, the dominant clinical feature at referral was AIM requiring immunosuppressive therapy. According to Bohan and Peter criteria, in 3 patients (LGL, BP, and AL) myositis could be classified as “definite polymyositis” whereas in patient DGP criteria for “possible polymyositis” were fulfilled.5 However, a more current diagnosis is overlap myositis, as recently defined.50 As shown in Table 1 , myositis was severe in patients LGL, BP, and AL, and less severe in patient DGP. In the former 3 patients, myositis was characterized by symmetric and proximal weakness with muscle strength graded 3/5 or 3.5/5 associated with elevated serum CK levels ranging from 1765 to 4836 U/L. Electromyography showed myositic abnormalities as defined by Bohan and Peter criteria.5 Quadriceps muscle biopsies, performed before the recent ENMC criteria,19 were consistent with idiopathic inflammatory myopathy as defined by Bohan and Peter.5 None of the patients was exposed to statin therapy.
Myositis in patients LGL, BP, and AL required high daily doses of prednisone (1 mg/kg), plus methotrexate (MTX) or azathioprine as a second immunomodulating agent to induce remission (see Table 1 ). In patients LGL and BP, the myositis was refractory, that is, initial corticosteroid therapy alone failed to induce remission, necessitating the addition of MTX (LGL) or azathioprine (BP).50 Given this aggressive profile of anti-NPC associated myositis, patient AL was treated from the outset with both corticosteroids and MTX. In all 3 patients, the myositis course was chronic, that is, requiring long-term corticosteroid treatment (daily prednisone >5 mg) and second-line therapy.50 Both patients LGL and AL experienced myositis relapses when prednisone was discontinued, necessitating reintroduction of this drug. The myositis course and therapy of patients LGL and BP are illustrated longitudinally in Figure 2.
FIGURE 2.
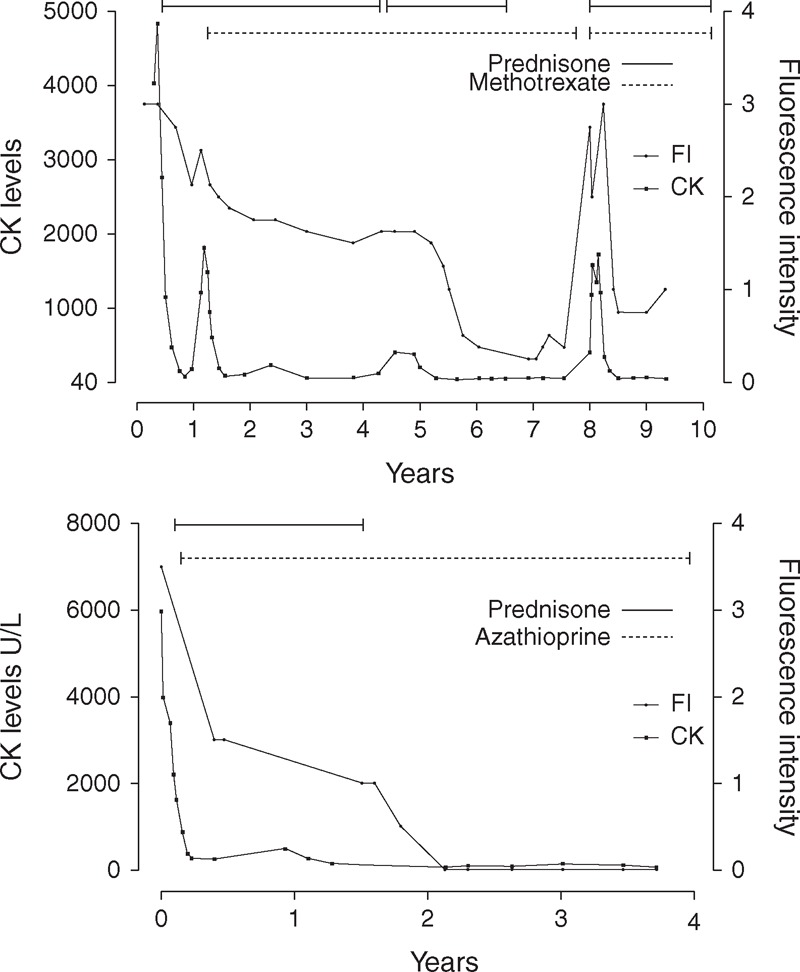
Longitudinal study reveals that titers of autoantibodies to nuclear pore complexes (anti-NPC) vary in parallel with myositis activity. Anti-NPC titers in consecutive and coded serum samples obtained from 2 patients over 10 and 4 years after myositis diagnosis, respectively, were measured by grading NPC fluorescence intensity (FI) on a scale of 0 to 4 at the screening dilution (1:40) on HEp-2 cells. FI was used as surrogate for titers and plotted with synchronous serum CK levels. Top panel. Patient LGL. The highest anti-NPC titer (FI of 3, endpoint titer 1:1280) correlated with the highest serum CK level (at year 0), followed by progressive fall and disappearance of anti-NPC with prednisone and methotrexate-induced remission (years 1 to 7). A lower titer of anti-NPC was persistently present from years 2 to 5, and then the autoantibody became undetectable. Immunosuppressive treatment discontinuation because of remission during year 7 was followed in late year 7 by a striking rise in anti-NPC titer and serum CK levels, together with a clinical myositis relapse, followed in year 8 by falling anti-NPC with treatment reinstitution and improving myositis. Bottom panel. Patient BP. The highest anti-NPC titer (FI of 3.5, endpoint titer 1:2560) correlated with the highest serum CK level (at year 0), followed by progressive fall of anti-NPC over 2 years and disappearance with sustained prednisone and azathioprine-induced myositis remission (years 2 to 4). No myositis relapse followed discontinuation of azathioprine after 5 years.
In the 4th patient (DGP), myositis was characterized by grade 4/5 proximal muscle weakness, oropharyngeal dysphagia, and mild serum aldolase and CK elevation. Remission was induced by prednisone 20 mg daily alone. No myositis relapse occurred over follow-up. Whether this favorable course was influenced by MTX introduced later for rheumatoid arthritis is unknown.
Other Overlap Syndrome Features Associated With Anti-NPC
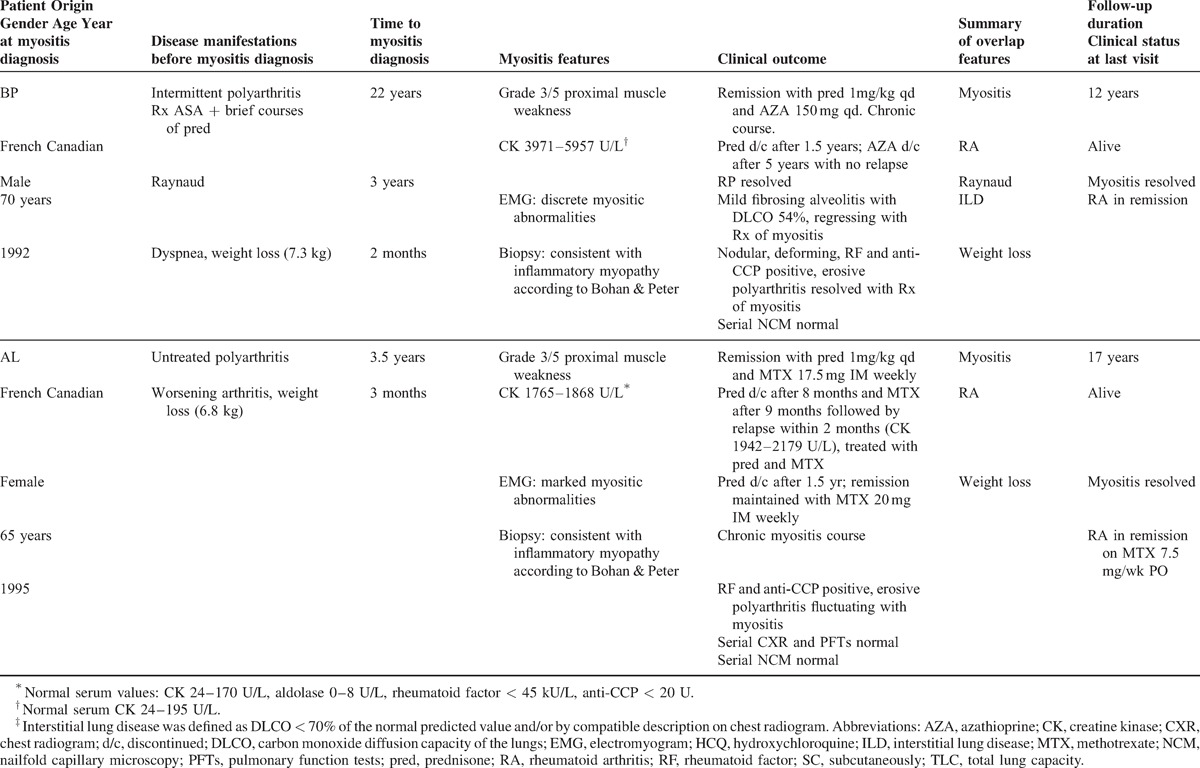
In addition to myositis, these patients shared several overlap syndrome features, including Raynaud phenomenon (n = 3 patients) and trigeminal neuropathy (n = 2 patients) (see Table 1 ). In patient DGP, trigeminal neuropathy preceded myositis by 4 years, whereas in patient LGL bilateral trigeminal neuropathy occurred during the 6 months preceding myositis. In 3 patients, weight loss (3.6–7.3 kg) preceded the diagnosis of myositis by several weeks.
Erosive, rheumatoid factor-positive, anti-CCP positive, and symmetric polyarthritis consistent with rheumatoid arthritis was present in 3 patients (BP, DGP, and AL) (see Table 1 ). In patient BP, rheumatoid arthritis was associated with olecranial nodules and ulnar deviation. In 2 patients, arthritis preceded myositis by several years, whereas in patient DGP, a cigarette smoker, arthritis onset occurred 3 years after myositis. Interstitial lung disease (n = 2) was mild and characterized by discrete fibrosing alveolitis on chest radiogram and pulmonary function tests showing a restrictive pattern for patient BP and a DLCO of 67% for patient DGP. A single patient, DGP, developed sicca symptoms with predominant xerophthalmia.
None of these patients showed features of SLE, or skin features of dermatomyositis or systemic sclerosis. Serial nailfold capillary microscopy remained normal in all patients.
Anti-NPC Overlap Syndrome May Be Associated With a Good Overall Prognosis
Although myositis required corticosteroids and a second immunomodulating agent for therapeutic control, myositis eventually subsided in all patients (see Table 1 ). No life-threatening systemic complication nor cancer have occurred at prolonged follow-up, with 100% survival at last visit (mean follow-up 19.5 yr, range 12–26 yr). Taken altogether, these data suggest that anti-NPC myositis-overlap syndrome may be associated with a good overall prognosis. However the number of patients reported herein is small and more patients will need to be studied.
Anti-NPC Parallel Myositis Activity
High titers of anti-NPC, ranging from 1:1280 to 1:10,240, were present at myositis diagnosis in sera from all 4 patients (Table 2). Anti-NPC titers fluctuated in parallel with the serum CK level in patients LGL, BP, and AL. This is illustrated in Figure 2 for patients LGL and BP. For both patients, the highest anti-NPC titer correlated with the highest CK level, followed by a progressive fall of anti-NPC to undetectable levels with treatment-induced remission. In patient AL, a similar decrease in anti-NPC titer in parallel with decreasing myositis activity was observed in consecutive serum samples over 5 months. Interestingly, in patient LGL a striking rise of anti-NPC coincided with clinical and enzymatic myositis relapse following discontinuation of prednisone and MTX because of remission (Figure 2A). In patient DGP, who was not treated with aggressive immunosuppression, anti-NPC titers remained elevated over time.
TABLE 1 (Continued).
Clinical manifestations before and at myositis diagnosis and long-term clinical outcome in 4 patients with autoantibodies to nuclear pore complexes
Anti-NPC Autoantibody Is Found Only in Overlap Myositis Sera
Anti-NPC were present in 4 (4%) patients of our cohort of 100 consecutive patients with AIM52 (Table 3). Anti-NPC were not found in sera from 393 adult patients with systemic sclerosis (n = 112), systemic lupus erythematosus (n = 94), rheumatoid arthritis (n = 45) (these diseases defined according to ACR criteria), MCTD (n = 35), autoimmune hepatitis (n = 13), psoriatic arthritis (n = 19), ankylosing spondylitis (n = 22), primary gout (n = 33), or primary osteoarthritis (n = 20), nor were they observed in sera from 62 normal adults. By 2-sided Fisher exact test, the association of anti-NPC with AIM was highly significant: for AIM versus all disease and normal controls, OR 42.4 (95% CI 2.3–796), p = 0.001; AIM vs disease controls only, OR 36.7 (95% CI 1.96–688), p = 0.0016.
TABLE 2.
Immunological profiles of 4 patients with autoantibodies to nuclear pore complexes (NPC) at myositis diagnosis
The Predominant Anti-NPC Isotype is IgG
The anti-NPC isotype present in all 4 patients at myositis diagnosis was IgG (see Table 2). In patients LGL and BP, only IgG anti-NPC was present. Patient DGP also expressed strong IgA anti-NPC, whereas patient AL expressed IgM as well as IgA anti-NPC, albeit at lower intensity than IgG.
Lack of Other IgG Autoantibody Connective Tissue Disease Marker in Anti-NPC Sera
No other IgG autoantibody marker for a defined connective tissue disease or to an overlap syndrome was identified in anti-NPC positive sera (see Table 2). Specifically, autoantibodies were absent to synthetases (Jo-1, PL-7, PL-12, OJ, EJ, KS, Tyr, and Zo), SRP, systemic sclerosis-specific autoantigens (CENP-B, topoisomerase I, RNA polymerase III, and Th/To), and to autoantigens associated with MCTD and systemic sclerosis or SLE in overlap (U1-U2-U3-U5RNP, PM-Scl, and Ku). Anti-p155/140, anti-MDA5, and anti-MJ were also negative.
Other Autoantibodies
Anticardiolipin and lupus anticoagulant antibodies were absent.
Heterogeneity of Nucleoporin Autoantigens
Nup358/RanBP2, a major component of the cytoplasmic filaments of the NPC is the autoantigen reactive with anti-NPC from patients DGP and AL (unpublished data and references53,54) (see Table 2). In addition, DGP serum reacted with gp210 but only by LIA. By immunoblotting, DGP serum did not react at all with gp210 and by immunogold electron microscopy, the NPC localization was typical of Nup358/RanBP2 and not that of gp210.53,54
LGL serum was previously shown to react by immunoblotting with polypeptides of 200 kD and 130 kD enriched in nuclear envelopes.10 In addition, LGL serum reacted by ALBIA with Nup62. Last, patient BP anti-NPC serum at a dilution of 1:500 reacted by immunoblotting with a single band of 90 kD protein in HeLa cells, rat cells and in Xenopus laevis and Pleurodele oocyte nuclear envelopes.9,27 By immunoelectron microscopy, anti-NPC from patient BP reacted with intranuclear filaments attached to NPCs. Taken together, these features suggested that patient BP serum recognized a novel nup, nup90, distinct from central domain-localized nup95 and intranuclear filament-associated nup153, as previously reported.9,27
Given that autoantibodies to gp210 and nup p62 have been associated with PBC, sera from the 4 patients were also tested for reactivity against a panel of autoantigens associated with autoimmune liver disease (see Table 2). All sera were negative except for DGP serum, which was positive for anti-mitochondrial M2 antibodies, but not for anti-M2–3E(BPO). Of interest, all patient sera were negative for antimitochondrial and anti-smooth muscle antibodies by indirect immunofluorescence on mouse tissues and for mitochondrial fluorescence on HEp-2 cells. Because of mildly elevated serum transaminases, and before detection of raised serum CK and the subsequent diagnosis of myositis, patient LGL had a liver biopsy, which was normal (see Table 1 ). Neither patient LGL nor patient DGP have shown any clinical or enzymatic evidence of liver disease over 26 years and 23 years of follow-up, respectively. In patient LGL, an abdominal ultrasound done 26 years after myositis diagnosis because of longstanding MTX therapy revealed no evidence of liver disease.
HLA Genotyping
By PCR-SSOP, the DQA1∗0501 allele was shared by all 4 patients (100%) with anti-nup (Table 4). The DPA1∗0103 allele was also shared by all patients. Three of the patients also carried the DRβ3∗0101 allele. The DRB1∗0301 allele was identified in 2 patients.
TABLE 3.
Frequency of autoantibodies to nuclear pore complexes in 493 adults with autoimmune and non autoimmune rheumatic diseases and 62 normal adults∗
DISCUSSION
To our knowledge, this is the first report describing the association between anti-NPC autoantibodies and an overlap syndrome with prominent myositis in a patient population of connective tissue diseases sharing a common ethnogeographic background. This clinical subset accounts for 4% of our cohort of French Canadian patients with AIM.50 Subsetting of patients with AIM according to their autoantibody markers is increasingly used by clinicians to diagnose more accurately these diseases, individualize their treatment and determine their prognosis.12,22,49 Thus, the shared phenotypic, immunogenetic and ethnogeographic characteristics in our patients with anti-NPC raise the question whether a novel AIM-overlap subset, the “anti-nup syndrome,” may be recognized by the presence of these autoantibodies.
Several data strongly support the concept of an “anti-nup syndrome” (Table 5). First, patients with a high titer of IgG anti-NPC autoantibodies share a similar clinical overlap syndrome. The 4 patients have in common prominent myositis as well as rheumatoid factor positive, anti-CCP positive and erosive rheumatoid arthritis, Raynaud phenomenon, mild interstitial lung disease and trigeminal neuralgia (see Table 5). The myositis features fulfilled proposed criteria for overlap myositis.50 At follow-up, no SLE manifestations or cutaneous features of dermatomyositis or systemic sclerosis were noted. Long-term survival was excellent and the overall prognosis was good.
TABLE 4.
MHC class II genotyping in 4 patients with autoantibodies to nuclear pore complexes
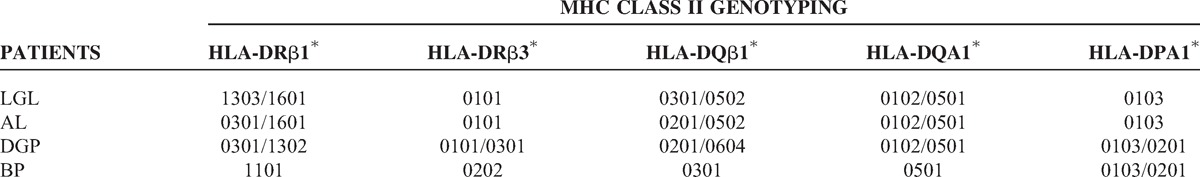
TABLE 5.
Summary of "anti-nup syndrome" features
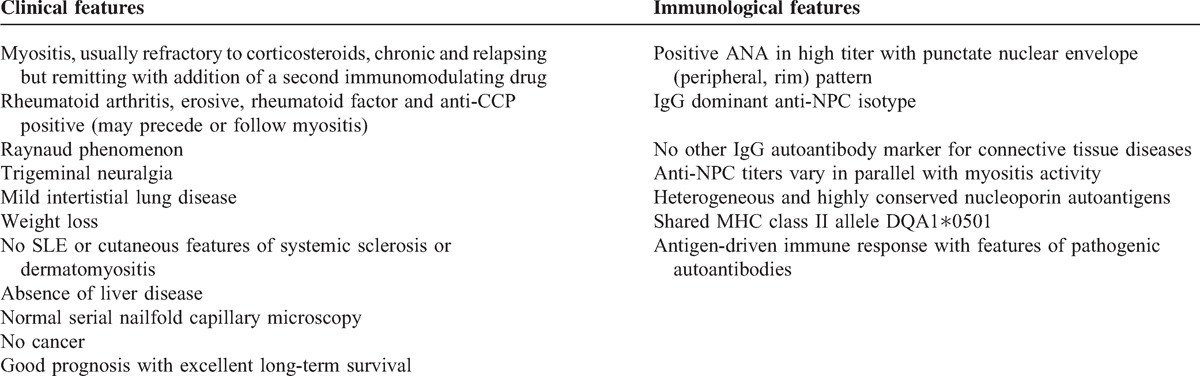
Second, the immunologic marker shared by these patients, that is, anti-NPC, appears selectively expressed, that is, no other myositis specific autoantibody, such as antisynthetases, nor any marker autoantibody associated with overlap myositis, such as anti-U1RNP, anti-U3RNP, anti-Ku, or anti-PM-Scl was identified.22,50 In fact, no other IgG autoantibody marker for a connective tissue disease was detected other than anti-NPC.
Third, all 4 patients shared the MHC Class II allele DQA1∗0501, the frequency of which is increased in white patients with AIM, most strikingly those with myositis specific autoantibodies.1,34,42 Two of the patients also carried a linked allele, DRB1∗0301. These unexpected findings suggest that patients with anti-NPC share immunogenetic disease susceptibility and, moreover, that shared and specific immunopathogenic mechanisms of disease are involved in these patients. Overall, the shared DQA1∗0501 allele brings strong pathophysiologic support to the concept of an “anti-nup syndrome.”
Fourth, anti-NPC titers varied strikingly in parallel with myositis activity, suggesting a mechanistic relationship, as yet undefined, to the pathophysiology of the disease. Interestingly, a similar fluctuation with myositis activity has been observed with anti-Jo-1 autoantibodies, which are markers for another overlap myositis syndrome, the antisynthetase syndrome.29
Last, the molecular heterogeneity of nup autoantigens is reminiscent of the antigenic heterogeneity observed in the antisynthetase syndrome, where autoantibodies to 8 synthetase autoantigens have been identified.46,49 None of the 4 patients with anti-nup displayed an antisynthetase. Moreover, there are major clinical differences between the clinical features in our patients and antisynthetase syndrome manifestations, including in the latter more severe interstitial lung disease, fever, dermatomyositis cutaneous features and a poorer prognosis with decreased survival.52 Thus, taken altogether, the data are consistent with an apparent “anti-nup syndrome” in our patient population.
This is the first report on anti-NPC autoantibodies encompassing a large group of patients from a uniform ethnogeographic background and with well-defined connective tissue diseases who were systematically screened by indirect immunofluorescence for the characteristic NPC fluorescent ANA pattern. Other reports are mostly case reports and have major methodologic differences with the present study, including absence of controls and lack of long-term follow-up. Anti-NPC were reported in 10 patients with connective tissue diseases from various ethnogeographic backgrounds.11,25,26,30 Anti-gp210 was noted in 2 patients with rheumatoid arthritis and Sjögren syndrome, respectively.25 Anti-p62 was reported in 4 patients with Sjögren syndrome,30 2 patients with rheumatoid arthritis,30 and a single MCTD patient with myositis requiring immunosuppressive treatment.26 Last, autoantibodies to Nup153 were reported in a single SLE patient.11 The few controlled studies are cross-sectional and focused on PBC and reactivity with a single nup. For example, in a French study of anti-gp210, 25% (n = 72) of 285 patients with PBC expressed the antibody whereas of 116 control patients with SLE, seropositive rheumatoid arthritis or systemic vasculitis, a single patient was positive for anti-gp210, for whom follow-up was not available.3
In summary, the relationship, if any, between these reports and the present study cannot be ascertained given methodologic differences, including lack of well-defined and large connective tissue disease control groups, testing restricted to reactivity with a single nup, lack of clinical phenotype description and/or absence of long-term follow-up. In addition, none of these patients was evaluated specifically for the anti-Nup358/RANBP2, anti-p90, or anti-p200/p130 specificities observed in the present study. Therefore, it will be important to study the frequency of anti-NPC as well as the associated phenotypes, antigenic specificities and immunologic features in other large international series of patients with connective tissue diseases, and particularly among patients with an overlap myositis syndrome.
Several nup specificities were identified by sera from our patients, indicating that the nup autoantigens are heterogeneous. Polypeptides of 200 kd and 130 kd were detected by LGL serum on immunoblots of nuclear envelope preparations.10 LGL serum also reacted with Nup62 by ALBIA.31,42 Nup358/RANBP2 was recognized by 2 patient sera (DGP and AL). A 180 kd polypeptide was initially identified as the autoantigen targeted by DGP serum and was called Nup180.53 Later, Nup180 was identified as a Nup358/RanBP2 fragment.54 Nup358/RanBP2 is a major component of the NPC cytoplasmic filaments and is essential for nuclear transport, mitosis and cell viability.16,54 In the search for the antigenic specificities associated with anti-NPC in our patients, we screened their sera for anti-gp210 and anti-Nup62. Interestingly, DGP serum (but not AL serum) reacted with gp210. However, by immunoblotting DGP serum did not react at all with gp210 and by immunogold electron microscopy, the NPC localization of Nup358/RanBP2 was distinct from gp210.53,54 Taken altogether, these data suggest that cross-reactive epitopes may account for the reactivity of DGP serum with gp210. Indeed, partial amino acid sequence homology was identified between Nup358/RanBP2 and gp210. For example, the Nup358/RanBP2 LLLK and SELAAL sequences are similar, respectively, to the gp210 LELK and STLAGL sequences.
The present report expands to the nuclear envelope, and specifically to the NPCs, the cellular compartments where autoantigens associated with overlap myositis syndromes are localized. Thus far, this had been restricted to the nucleus and to the cytoplasm. Autoantibodies to the nuclear autoantigens U1RNP, U3RNP, Ku and PM-Scl are associated with various overlap myositis syndromes. In contrast, autoantibodies to the cytoplasmically localized synthetases and MDA5 autoantigens are associated, respectively, with the antisynthetase syndrome and with a dermatomyositis overlap syndrome. The latter syndromes are phenotypically distinct from the former ANA-associated syndromes and, in turn, appear distinct from anti-nup syndrome features,15,49 although a larger number of patients with connective tissue diseases and anti-nup autoantibodies will need to be studied.
The autoantigens identified by our patient sera are also intriguing in light of the differential localization of these proteins within the NPC structure. Nup358/RanBP2 is found at the cytoplasmic face of the NPC while gp210 is part of the central framework.18a,27 Nup62 is a complex of proteins (p62, p60, and p54) also localized in the central framework composing the central channel.18a,21 In contrast, Nup90, identified using anti-NPC serum from patient BP, localizes at the intranuclear NPC-attached filaments.27 The exact relationship between the molecular identity and cellular localization of these autoantigens and the corresponding overlap myositis syndromes remains to be deciphered.
Several features of the autoimmune response that characterizes anti-NPC autoantibodies in our patients deserve comment (see Table 5). Anti-NPC were predominantly of IgG isotype and present in high titers at diagnosis. Anti-NPC varied in parallel with myositis activity or persisted in high titers over time and were directed to highly conserved antigenic determinants within the cytoplasmic, nuclear or central framework of the NPC. Taken together, these features suggest that nup autoantigens themselves drive the anti-nup autoimmune response. The absence of other connective tissue disease marker autoantibodies is consistent with antigen selectivity and a highly focused immune response. Thus, anti-nup autoantibodies appear antigen-driven and display some features of pathogenic autoantibodies.32
The initiating event that trigger anti-nup immune responses is unknown. In PBC, molecular mimicry following hepatitis B virus infection was evoked as a potential mechanism, as certain nup amino acid sequences display similarity with hepatitis B virus DNA polymerase.13 However, PBC patient sera did not react with the shared epitopes.13 None of our 4 patients with anti-NPC had serologic evidence of hepatitis B virus infection. Mimicry of bacterial antigenic determinants has also been proposed since the predominant autoepitope of gp210 recognized by anti-gp210 from 25 PBC patients is homologous to Escherichia coli mut Y and Salmonella typhimurium mut B gene products.33 However, further study revealed that mut Y gene product was not recognized by anti-gp210 autoantibodies.8
Finally, although no direct mechanistic link between anti-NPC and myositis is established, a specific pathophysiologic link between nups and striated skeletal muscle is suggested by the correlation between anti-NPC titers and myositis activity. Interestingly, other nuclear envelope components are known to be critical in muscle disease. For example, emerin and lamin A/C gene mutations are associated with specific muscular dystrophies.7,44 Furthermore, Asally et al2 have identified a key role for Nup358 in skeletal myogenesis. More studies are needed to clarify the pathophysiologic link between anti-nup autoantibodies and the “anti-nup syndrome.”
ACKNOWLEDGMENTS
The authors thank Gemma Perez and Haiyan Hou for laboratory assistance and Carole Blouin for secretarial assistance.
Footnotes
Abbreviations: ACR = American College of Rheumatology, AIM = autoimmune myositis, ALBIA = addressable laser bead immunoassay, ANAs = autoantibodies to nuclear antigens, CENP-B = centromere protein B, CK = creatine kinase, CHUM = Centre Hospitalier de l’Université de Montréal, DLCO = carbon monoxide lung diffusing capacity, ELISA = enzyme-linked immunosorbent assay, ENMC = European Neuromuscular Centre, LC-1 = liver cytosolic-1 antigen, LIA = line immunoassay, LKM-1 = liver-kidney-microsome-1, MCTD = mixed connective tissue disease, MDA-5 = melanoma differentiation-associated protein 5, MTX = methotrexate, NPCs = nuclear pore complexes, NUPs = nucleoporins, OM = overlap myositis, PBC = primary biliary cirrhosis, PML = promyelocytic leukemia cell antigen, SLA/LP = soluble liver antigen/liver pancreas antigen, SLE = systemic lupus erythematosus, RNP = ribonucleoprotein, SRP = signal recognition particle, SumoAE = small ubiquitin-like modifier activating enzyme, TRIM21 = tripartite motif.
Financial support and conflicts of interest: This study was supported in part by operating grants MT-14636, MOP-62687, and MOP-81252 from the Canadian Institutes of Health Research (to JLS), by Department of Veterans Affairs Medical Research Funds (to INT), and by donations from Sclérodermie Québec and Mrs Gisèle Sarrazin-Locas in support of The Laboratory for Research in Autoimmunity. JLS holds the University of Montreal Scleroderma Research Chair. MJF holds the Arthritis Society Research Chair at the University of Calgary. Authors JLS, CI, MCD, RG, and FJ have no conflicts of interest to disclose. The authors listed below have received financial support (personal or institutional) from the listed institutions or pharmaceutical companies, unrelated to the present work. MJF: EUROIMMUN, ImmunoConcepts, INOVA Diagnostics; INT: Oklahoma Medical Research Foundation Clinical Immunology Laboratory, UpToDate; YT: Abbvie, Amgen, BMS, Janssen, Roche, UCB, Warner-Chilcott; MG: Novartis; JPR: Arthrolab, Amgen, BMS, Cellgene, Janssen, Lilly, Roche, Pfizer.
REFERENCES
- 1.Arnett FC, Targoff IN, Mimori T, et al. Interrelationship of major histocompatibility complex class II alleles and autoantibodies in four ethnic groups with various forms of myositis. Arthritis Rheum. 1996; 39:1507–1518. [DOI] [PubMed] [Google Scholar]
- 2.Asally M, Yasuda Y, Oka M, et al. Nup358, a nucleoporin, functions as a key determinant of the nuclear pore complex structure remodeling during skeletal myogenesis. FEBS Journal. 2011; 278:610–621. [DOI] [PubMed] [Google Scholar]
- 3.Bandin O, Courvalin JC, Poupon R, et al. Specificity and sensitivity of gp210 autoantibodies detected using an enzyme-linked immunosorbent assay and a synthetic polypeptide in the diagnosis of primary biliary cirrhosis. Hepatology. 1996; 23:1020–1024. [DOI] [PubMed] [Google Scholar]
- 4.Benveniste O, Drouot L, Jouen F, et al. Correlation of anti-signal recognition particle autoantibody levels with creatine kinase activity in patients with necrotizing myopathy. Arthritis Rheum. 2012; 63:1961–1971. [DOI] [PubMed] [Google Scholar]
- 5.Bohan A, Peter JB. Polymyositis and dermatomyositis (parts 1 and 2). N Engl J Med. 1975; 292:344–347.403-407. [DOI] [PubMed] [Google Scholar]
- 6.Capelson M, Hetzer MW. The role of nuclear pores in gene regulation, development and disease. EMBO Reports. 2009; 10:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi Y-H, Chen ZJ, Jeang KT. The nuclear envelopathies and human diseases. J Biomed Sci. 2009; 16:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courvalin JC, Worman HJ. Nuclear envelope protein autoantibodies in primary biliary cirrhosis. Sem Liv Dis. 1997; 17:79–90. [DOI] [PubMed] [Google Scholar]
- 9.Dabauvalle MC, Wilken N, Ewald A. Jouffrey B, Colliex C, et al. Nuclear pore complex structure analyzed by immunogold EM with human autoantibodies. Electron microscopy 1994, Applications in Biological Sciences. Paris, France:Editions de Physique; 1994; Vol. 3A, 475–478. [Google Scholar]
- 10.Dagenais A, Bibor-Hardy V, Senecal JL. A novel autoantibody causing a peripheral fluorescent antinuclear antibody pattern is specific for nuclear pore compexes. Arthritis Rheum. 1988; 31:1322–1327. [DOI] [PubMed] [Google Scholar]
- 11.Enarson P, Rattner JB, Ou Y, et al. Autoantigens of the nuclear pore complex. J Mol Med. 2004; 82:423–433. [DOI] [PubMed] [Google Scholar]
- 12.Fiorentino D, Casciola-Rosen L. Autoantibodies to transcription intermediary Factor 1 in dermatomyositis shed insight into the cancer-myositis connection. Arthritis Rheum. 2012; 64:346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregorio GV, Choudhuri K, Ma Y, et al. Mimicry between the hepatitis B virus DNA polymerase and the antigenic targets of nuclear and smooth muscle antibodies in chronic hepatitis B virus infection. J Immunol. 1999; 162:1802–1810. [PubMed] [Google Scholar]
- 14.Grunwald D, Singer RH, Rout M. Nuclear export dynamics of RNA-protein complexes. Nature. 2011; 475:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall JC, Casciola-Rosen L, Samedy LA, et al. Anti–melanoma differentiation–associated protein 5–associated dermatomyositis: expanding the clinical spectrum. Arthritis Care Res. 2013; 65:1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamada M, Haeger A, Jeganathan KB, et al. Ran-dependent docking of importin-β to RanBP2/Nup358 filaments is essential for protein import and cell viability. J Cell Biol. 2011; 4:597–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henault J, Tremblay M, Clement I, et al. Direct binding of anti-DNA topoisomerase I antibody to the cell surface of fibroblasts in patients with systemic sclerosis. Arthritis Rheum. 2004; 50:3265–3274. [DOI] [PubMed] [Google Scholar]
- 18.Hetzer MW, Wente SR. Border control at the nucleus: biogenesis and organization of the nuclear membrane and pore complexes. Developmental Cell. 2009; 17:606–616.18a Hoelz A, Debler EW, Blobel G. The structure of the nuclear pore complex. Annu Rev Biochem. 2011;80:613–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoogendijk JE, Amato AA, Lecky BR, et al. 119th ENMC international workshop: Trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, The Netherlands. Neuromuscular Disorders. 2004; 14:337–345. [DOI] [PubMed] [Google Scholar]
- 20.Imreh G, Hallberg E. An integral membrane protein from the nuclear pore complex is also present in the annulate lamellae: implications for annulate lamella formation. Exp Cell Res. 2000; 259:180–190. [DOI] [PubMed] [Google Scholar]
- 21.Kita K, Omata S, Horigome T. Purification and characterization of a nuclear pore glycoprotein complex containing p62. J Biochem. 1993; 113:377–382. [DOI] [PubMed] [Google Scholar]
- 22.Koenig M, Fritzler MJ, Targoff IN, et al. Heterogeneity of autoantibodies in 100 patients with autoimmune myositis: insights into clinical features and outcomes. Arthritis Res Ther. 2007; 9:R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koenig M, Dieude, Senecal JL. Predictive value of antinuclear autoantibodies: the lessons of the systemic sclerosis autoantibodies. Autoimmunity Reviews. 2008; 7:588–593. [DOI] [PubMed] [Google Scholar]
- 24.Koenig M, Joyal F, Fritzler MJ, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud's phenomenon to systemic sclerosis: a twenty-year prospective study of 586 Patients, with validation of proposed criteria for early systemic sclerosis. Arthritis Rheum. 2008; 58:3902–3912. [DOI] [PubMed] [Google Scholar]
- 25.Konstantinov K, Foisner R, Byrd D, et al. Integral membrane proteins associated with the nuclear lamina are novel autoimmune antigens of the nuclear envelope. Clin Immunol Immunopathol. 1995; 74:89–99. [DOI] [PubMed] [Google Scholar]
- 26.Kraemer DM, Kraus MR, Kneitz C, Tony H-P. Nucleoporin p62 antibodies in a case of mixed connective tissue disease. Clin Diagn Lab Immunol. 2003; 10:329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhbier A, Ewald A, Senecal JL, et al. p90, a novel protein of the intranuclear filaments attached to nuclear pore complex. Proceedings of the 29th Paterson Symposium Manchester, England; November 1994. [Google Scholar]
- 28.Liang Y, Hetzer MW. Functional interactions between nucleoporins and chromatin. Curr Opin Cell Biol. 2011; 23:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller FW, Waitet KA, Biswasf T, Plotz P. The role of an autoantigen, histidyl-tRNA synthetase, in the induction and maintenance of autoimmunity. Proc Natl Acad Sci USA. 1990; 87:9933–9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyachi K, Shibata M, Onozuka Y, et al. Primary biliary cirrhosis sera recognize not only gp210 but also proteins of the p62 complex bearing N-acetylglucosamine residues from rat liver nuclear envelope. Anti-p62 complex antibody in PBC. Mol Biol Rep. 1996; 23:227–234. [DOI] [PubMed] [Google Scholar]
- 31.Miyachi K, Hankins RW, Matsushima H, et al. Profile and clinical significance of anti-nuclear envelope antibodies found in patients with primary biliary cirrhosis: a multicenter study. J Autoimmunity. 2003; 20:247–254. [DOI] [PubMed] [Google Scholar]
- 32.Naparstek Y, Plotz PH. The role of autoantibodies in autoimmune disease. Annu Rev Immunol. 1993; 11:79–104. [DOI] [PubMed] [Google Scholar]
- 33.Nickowitz RE, Worman HJ. Autoantibodies from patients with primary biliary cirrhosis recognize a restricted region within the cytoplasmic tail of nuclear pore membrane glycoprotein gp210. J Exp Med. 1993; 178:2237–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Hanlon TP, Mercatante Carrick D, Targoff IN, et al. Immunogenetic risk and protective factors for the idiopathic inflammatory myopathies - Distinct HLA-A, -B, -Cw, -DRB1, and -DQA1 allelic profiles distinguish European American patients with different myositis autoantibodies. Medicine (Baltimore). 2006; 85:111–127. [DOI] [PubMed] [Google Scholar]
- 35.Podrebarac TA, Boisvert DM, Goldstein R. Clinical correlates, serum autoantibodies and the role of the major histocompatibility complex in French Canadian and non-French Canadian Caucasians with SLE. Lupus. 1998; 7:183–191. [DOI] [PubMed] [Google Scholar]
- 36.Santiago M, Baron M, Miyachi K. The Canadian Scleroderma Research Group, Fritzler MJ. A comparison of the frequency of antibodies to cyclic citrullinated peptides using a third generation anti-CCP assay (CCP3) in systemic sclerosis, primary biliary cirrhosis and rheumatoid arthritis. Clin Rheumatol. 2008; 27:77–83. [DOI] [PubMed] [Google Scholar]
- 37.Scussel-Lonzetti L, Joyal F, Raynauld J-P, et al. Predicting mortality in systemic sclerosis. analysis of a cohort of 309 French Canadian patients with emphasis on features at diagnosis as predictive factors for survival. Medicine (Baltimore). 2002; 81:154–167. [DOI] [PubMed] [Google Scholar]
- 38.Senecal J-L, Oliver JM, Rothfield N. Anticytoskeletal autoantibodies in the connective tissue diseases. Arthritis Rheum. 1985; 28:889–898. [DOI] [PubMed] [Google Scholar]
- 39.Senecal J-L, Fortin S, Roussin A, Joyal F. Anticytoskeletal autoantibody to microfilament anchorage sites recognizes novel focal contact proteins. J Clin Invest. 1987; 80:778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Senecal JL, Raymond Y. Autoantibodies to DNA, lamins, and pore complex proteins produce distinct peripheral fluorescent antinuclear antibody patterns on the HEp-2 substrate. Arthritis Rheum. 1991; 34:249–251. [DOI] [PubMed] [Google Scholar]
- 41.Senecal J-L, Rauch J, Grodzicky T, et al. Strong association of autoantibodies to human nuclear lamin B1 with lupus anticoagulant antibodies in systemic lupus erythematous. Arthritis Rheum. 1999; 42:1347–1353. [DOI] [PubMed] [Google Scholar]
- 42.Shamim EA, Rider LG, Miller FW. Update on the genetics of the idiopathic inflammatory myopathies. Curr Opin Rheumatol. 2000; 12:482–491. [DOI] [PubMed] [Google Scholar]
- 43.Stinton LM, Swain M, Myers RP, et al. Autoantibodies to GW bodies and other autoantigens in primary biliary cirrhosis. Clin Exp Immunol. 2011; 163:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimi T, Butin-Israeli V, Goldman RD. The functions of the nuclear envelope in mediating the molecular crosstalk between the nucleus and the cytoplasm. Current Opin Cell Biol. 2012; 24:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Targoff IN. Autoantibodies to aminoacyl-transfer RNA synthetases for isoleucine and glycine: two additional synthetases are antigenic in myositis. J Immunol. 1990; 144:1737–1743. [PubMed] [Google Scholar]
- 46.Targoff IN. Autoantibodies in polymyositis. Rheum Dis Clin North Am. 1992; 18:455–482. [PubMed] [Google Scholar]
- 47.Targoff IN, Trieu EP, Miller FW. Reaction of anti-OJ autoantibodies with components of the multi-enzyme complex of aminoacyl-tRNA synthetases in addition to isoleucyl-tRNA synthetase. J Clin Invest. 1993; 91:2556–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Targoff IN, Mamyrova G, Trieu EP, et al. for the Childhood Myositis Heterogeneity and International Myositis Collaborative Study Groups A novel autoantibody to a 155-kd protein is associated with dermatomyositis. Arthritis Rheum. 2006; 54:3682–3689. [DOI] [PubMed] [Google Scholar]
- 49.Targoff IN. Autoantibodies and their significance in myositis. Curr Rheumatol Rep. 2008; 10:333–340. [DOI] [PubMed] [Google Scholar]
- 50.Troyanov Y, Targoff IN, Tremblay JL, et al. Novel classification of idiopathic inflammatory myopathies based on overlap syndrome features and autoantibodies. Analysis of 100 French Canadian patients. Medicine (Baltimore). 2005; 84:231–249. [DOI] [PubMed] [Google Scholar]
- 51.Vazquez-Abad D, Wallace S, Senecal JL, et al. Anti-centromere antibodies. Evaluation of an ELISA using recombinant fusion protein CENP-B as antigen. Arthritis Rheum. 1994; 37:248–252. [DOI] [PubMed] [Google Scholar]
- 52.Venables PJW. Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH. Overlap syndromes. Rheumatology. Philadelphia, PA:Mosby Elsevier; 2011. 1491–1497. [Google Scholar]
- 53.Wilken N, Kossner U, Senecal J-L, et al. Nup180, a novel nuclear pore complex protein localizing to the cytoplasmic ring and associated fibrils. J Cell Biol. 1993; 123:1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilken N, Senecal J-L, Scheer U, Dabauvalle M-C. Localization of the Ran-GTP binding protein RanBP2 at the cytoplasmic side of the nuclear pore complex. Eur J Cell Biol. 1995; 68:211–219. [PubMed] [Google Scholar]
- 55.Worman HJ, Courvalin J-C. Antinuclear antibodies specific for primary biliary cirrhosis. Autoimmunity Reviews. 2003; 2:211–217. [DOI] [PubMed] [Google Scholar]


