Supplemental Digital Content is available in the text
Abstract
Carbon monoxide (CO) poisoning is considered one of the most crucial health concerns. Few studies have investigated the correlation between CO poisoning and the risk of developing cardiovascular diseases (CVDs). Therefore, we conducted a population-based, longitudinal cohort study in Taiwan to determine whether patients with CO poisoning are associated with higher risk of developing subsequent CVDs, including arrhythmia, coronary artery disease (CAD) and congestive heart failure (CHF).
This retrospective study used the National Health Insurance Research Database. The study cohort comprised all patients aged ≥20 years with a diagnosis of CO poisoning and hospitalized during 2000 to 2011 (N = 8381), and the comparison cohort comprised randomly selected non-CO-poisoned patients (N = 33,524) frequency-matched with the study cohort by age, sex, and the year of index date. Each patient was individually tracked to identify those who develop CVD events during the follow-up period. Cox proportional hazards regression model was performed to calculate the hazard ratios of CVDs after adjusting for possible confounders.
The overall incidences of arrhythmia, CAD, and CHF were higher in the patients with CO poisoning than in the controls (2.57 vs 1.25/1000 person-years, 3.28 vs 2.25/1000 person-years, and 1.32 vs 1.05/1000 person-years, respectively). After adjusting for age, sex, and comorbidities, the patients with CO poisoning were associated with a 1.83-fold higher risk of arrhythmia compared with the comparison cohort, and nonsignificantly associated with risk of CAD and CHF. CO-poisoned patients with coexisting comorbidity or in high severity were associated with significantly and substantially increased risk of all 3 CVDs.
CO poisoning is associated with increased risk of subsequent development of arrhythmia. Future studies are required to explore the long-term effects of CO poisoning on the cardiovascular system.
INTRODUCTION
For the past decades, carbon monoxide (CO) poisoning, the so-called “silent killer,” has been one of the most crucial health concerns worldwide, primarily because of its severe clinical effects and high toxicological morbidity and mortality.1 It accounts for 50,000 emergency department visits and 2700 deaths annually in the United States.2 CO is an odorless and colorless gas generated because of incomplete combustion of carbon-containing fuels. The mechanism of CO toxicity is tissue hypoxia, since the binding affinity of CO to hemoglobin is 200 to 240 times that of oxygen, which reduces oxygen-carrying capacity and impairs the release of oxygen to tissues.
CO affects nearly all the organs and tissues, but the toxidromes lack clinical specificity and are often overlooked or misdiagnosed.3,4 In fact, the high oxygen demand of the cardiovascular and central nervous systems causes them to predominate the acute and delayed clinical features.5 Cardiovascular disease represents the leading cause of death in the United States and around the world, which accounts for approximately 30% of all deaths.6 Previous studies have focused on the cardiac dysfunction related to CO poisoning and indicated that myocardial injury is frequent in moderate-to-severe CO poisoning.1,7–11 However, based on a review of the literature, only limited case reports have confirmed the effects of CO toxicity on the cardiovascular system. To obtain sufficient statistical power, we used a large-scale database and sought to investigate the correlation between CO poisoning and the subsequent development of major cardiovascular diseases (CVDs) in Taiwanese patients with no history of CVD.
METHODS
Data Source
We used the National Health Insurance Research Database (NHIRD) released by the Taiwan National Health Research Institutes. The National Health Insurance (NHI) system is a mandatory universal health insurance program that offers comprehensive medical care coverage to all Taiwan residents. According to the NHI annual statistics report, in 2007, the NHI covered approximately 99% of the entire population of Taiwan, and over 25 million people were enrolled in this program (http://www.nhi.gov.tw/english/index.aspx). International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes were used for identifying the diseases of interest in the study.
Sampled Patients
Based on the inpatient claims, patients who were hospitalized for CO poisoning (ICD-9-CM code 986) between January 1, 2000 and December 31, 2011, with no medical history of arrhythmia (ICD-9-CM codes 427), coronary artery disease (CAD) (ICD-9-CM codes 410–414), congestive heart failure (CHF) (ICD-9-CM code 428), and complete age or sex information, were enrolled in the study. The first hospitalization dates for CO poisoning were defined as the index dates. Overall 8381 patients with CO poisoning comprised the study cohort. The comparison cohort comprised 4 non-CO-poisoned control patients for each CO-poisoned patient in the study cohort, frequency-matched by age with an interval of 5 years, sex, and the year of index date. The control patients with a history of arrhythmia, CAD, and CHF before the index date, or incomplete age or sex information, were excluded and replaced with corresponding qualifying patients. Finally, 33,524 non-CO-poisoned control patients formed the comparison cohort of the study.
Outcome and Comorbidities
The CO-poisoned (study) and the non-CO-poisoned (comparison) cohorts were followed up until the diseases appeared or they were censored because of loss to follow-up, death, or the end of 2011, whichever occurred earlier, to measure the incidence of arrhythmia, CAD, and CHF. A history of diabetes (ICD-9-CM code 250), hypertension (ICD-9-CM codes 401–405), hyperlipidemia (ICD-9-CM code 272), and chronic obstructive pulmonary disease (COPD) (ICD-9-CM codes 490–492, 494, 496) was considered as a comorbidity. In addition, acute respiratory failure (ICD-9-CM code 518.81) was considered a severity indicator based on diagnoses in the hospitalization records since the index date and within the first 3 days.
Ethics Statement
The study performed conform the declaration of Helsinki. Informed consent was not required for the inclusion of these patients because the NHIRD encrypts patient’ personal information to protect privacy and provides researchers with anonymous identification numbers associated with relevant claim information, including patients’ sex, dates of birth, medical services utilized, and prescriptions. This study was approved by the Institutional Review Board of China Medical University (CMU-REC-101–012). Our IRB specifically waived the consent requirement.
Statistical Analysis
Data analyses compared the distributions of age, sex, and baseline comorbidities between the 2 cohorts. The χ2 test was used to examine the categorical variables, whereas the t test was used to examine the continuous variables. The incidence of arrhythmia, CAD, and CHF were identified in each cohort. The follow-up time in person-years was used to estimate the incidence density rates. Univariate and multivariate Cox proportional hazard regressions were used to determine the effects of CO poisoning on the risk of arrhythmia, CAD, and CHF and indicated by the hazard ratio (HR) with a 95% confidence interval (CI). A multivariate model was simultaneously adjusted for sex, age, and the comorbidities of diabetes, hypertension, hyperlipidemia, and COPD. Subsequently, we evaluated the risk variance over time by stratifying the follow-up period into 3 segments: ≤3, 4 to 6, and >6 years. All analyses were performed using SAS (Version 9.3, SAS Institute Inc, Cary, NC, USA), with 2-sided P values <0.05 considered statistically significant.
RESULTS
Table 1 summarizes the distributions of the demographic variables and comorbidities for both the groups. The mean (±standard deviation) age of the study and comparison cohorts was 38.8 (±13.0) and 38.7(±13.4) years, respectively. The study cohort patients exhibited a higher prevalence of comorbidities than did the comparison cohort patients (all P < .001).
TABLE 1.
Characteristics of Patients With Carbon Monoxide Poisoning and Matched Patients Without Carbon Monoxide Poisoning
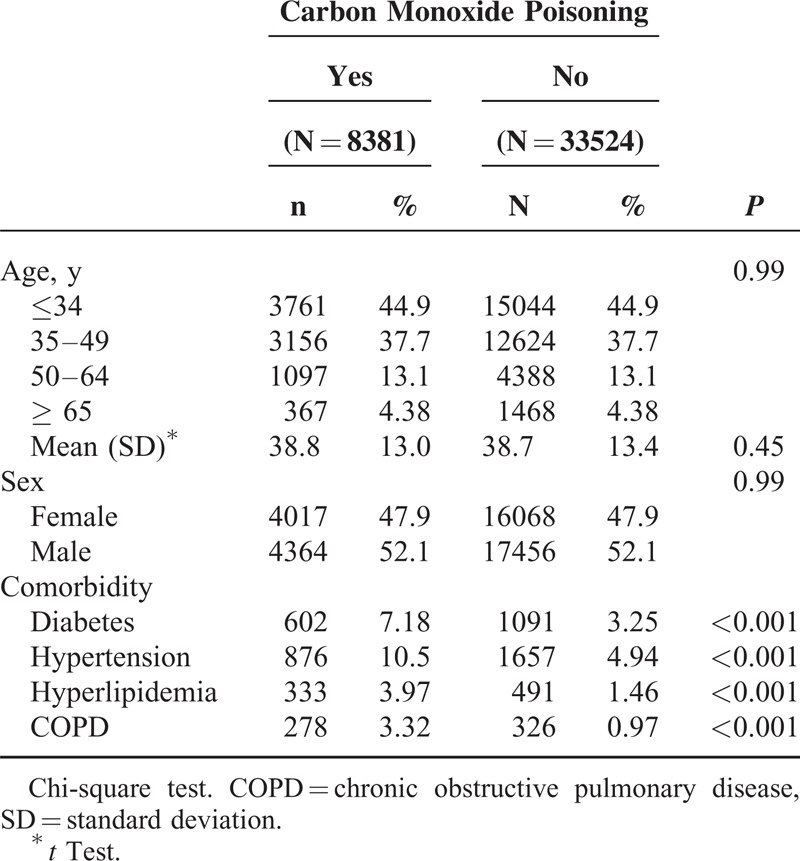
Overall, the incidence of arrhythmia was 2.06-fold higher in the study cohort than in the comparison cohort (2.57 vs 1.25 per 1000 person-years), with an adjusted HR of 1.83 (95% CI = 1.43–2.33) (Table 2). The patients in the youngest age group exhibited the highest age-specific relative risk (adjusted HR = 3.47; 95% CI = 2.46–4.91). The risk of arrhythmia was 2.83-fold higher in the study cohort patients with no comorbidity than in the corresponding comparison cohort patients (95% CI = 1.98–4.04). The overall incidence of CAD was not significantly higher in the study cohort than in the comparison cohort (3.28 vs 2.25 per 1000 person-years), with an adjusted HR of 1.14 (95% CI = 0.93–1.40). In addition, the highest risk for CAD was observed in female patients (adjusted HR = 1.60, 95% CI = 1.17–2.20), patients aged ≤49 years (adjusted HR = 1.47, 95% CI = 1.08–1.99) and those with no comorbidities (adjusted HR = 1.56, 95% CI = 1.20–2.02). However, no significant differences were observed in the overall incidence density of CHF between the study and the comparison cohorts (1.32 vs 1.05 per 1000 person-years).
TABLE 2.
Incidence and Hazard Ratio of Arrhythmia, Coronary Artery Disease, and Congestive Heart Failure Between Patients with Carbon Monoxide Poisoning and Without Carbon Monoxide Poisoning
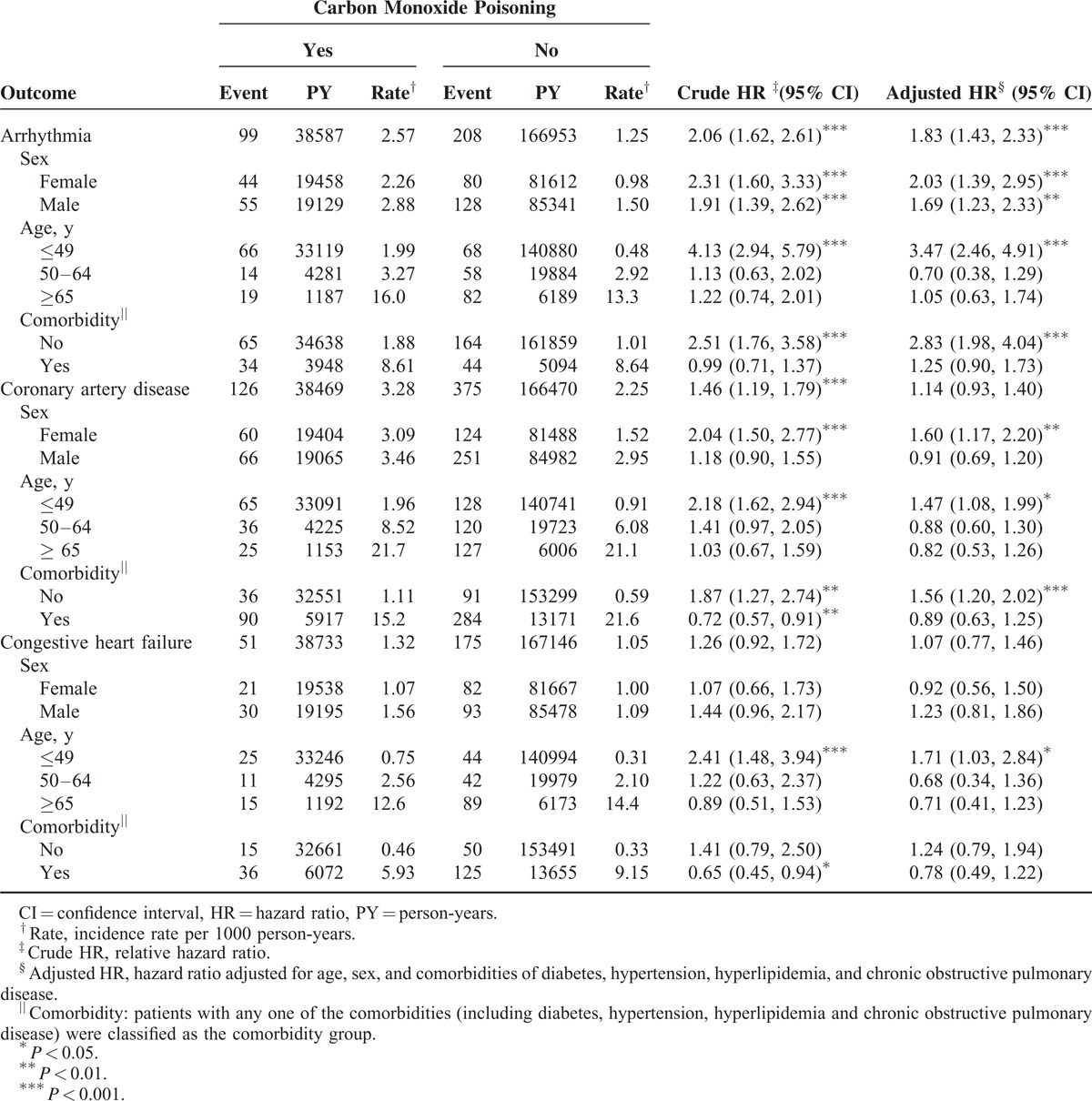
Table 3 lists the interaction effects of CO poisoning and comorbidity on the risks of cardiovascular diseases. The study cohort patients with comorbidities exhibited a significantly higher risk of arrhythmia (adjusted HR = 7.51, 95% CI = 5.25–10.7; P < 0.001) than the corresponding cohort patients with comorbidities. Moreover, the study cohort patients with comorbidities exhibited a 14.7-fold higher risk of CAD than did the corresponding comparison cohort patients without comorbidities (95% CI = 10.9–19.9; P < 0.001).
TABLE 3.
Cox Proportional Hazard Regression Analysis for the Risk of Cardiovascular Event-associated Carbon Monoxide Poisoning With Interaction of Comorbidity
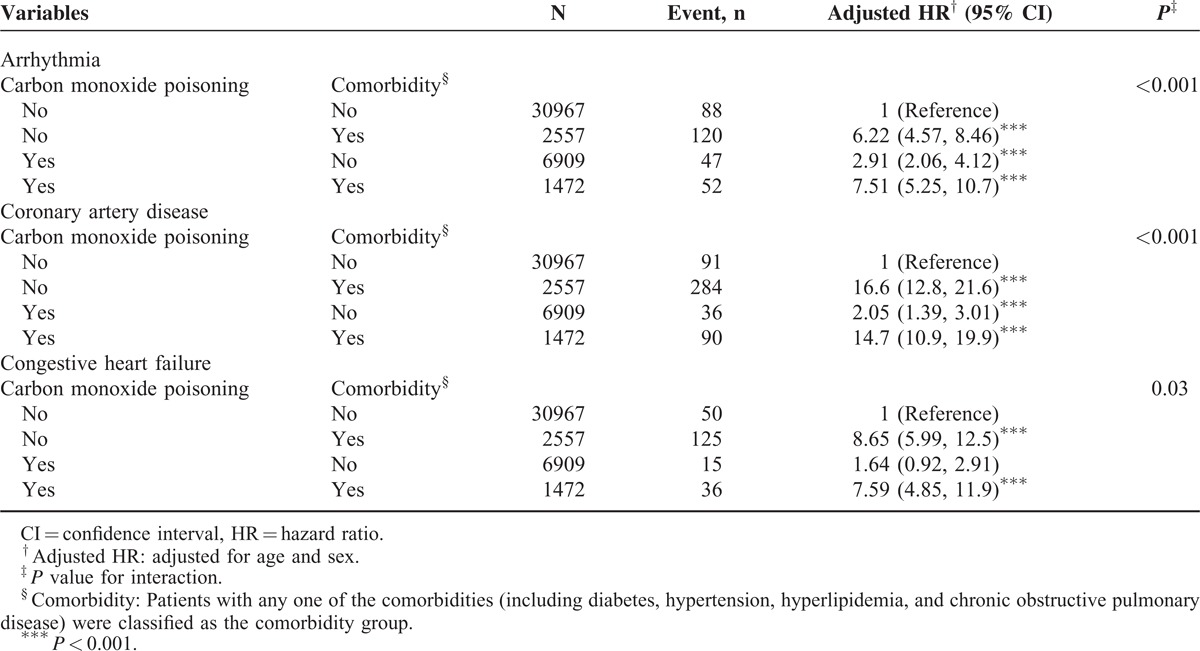
Furthermore, relative to the comparison cohort, the study cohort patients with severe CO poisoning exhibited a significantly much higher risk of arrhythmia (HR = 4.41, 95% CI = 2.87–6.76) than did those with less severe CO poisoning (HR = 1.56, 95% CI = 1.20–2.04) (Table 4). Patients with severe CO poisoning exhibited a higher risk of CAD (adjusted HR = 1.75, 95% CI = 1.06–2.89) and CHF (adjusted HR = 3.01, 95% CI = 1.73–5.23) than did those without CO poisoning.
TABLE 4.
Cox Proportional Hazard Regression Analysis for the Risk of Cardiovascular Event Stratified by the Severity of Carbon Monoxide Poisoning
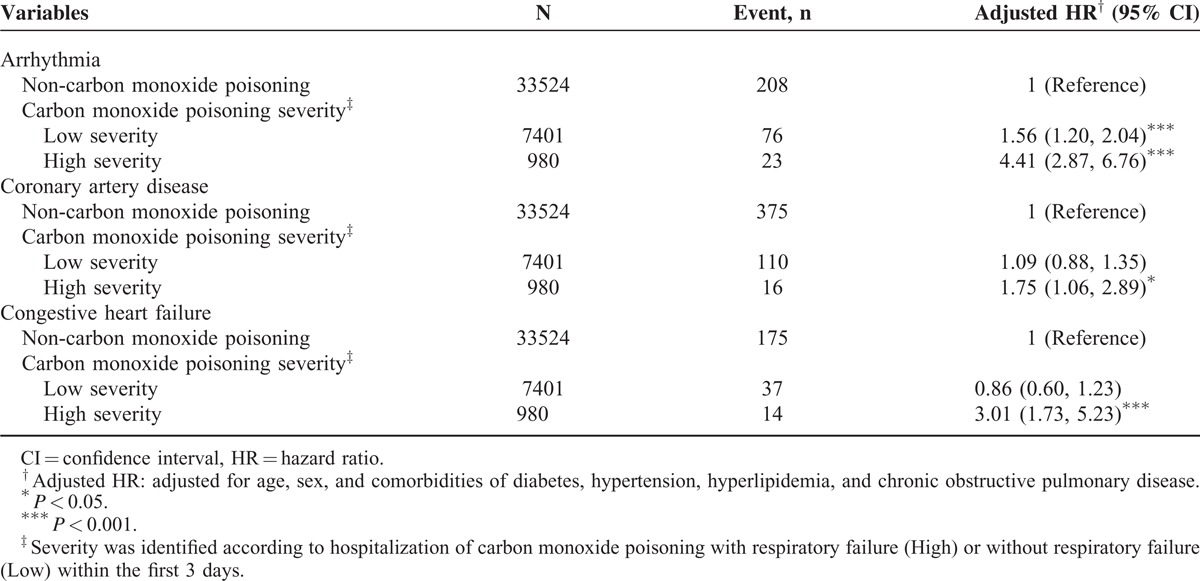
Table 5 summarizes the comparisons of the risks of cardiovascular events by stratifying the follow-up periods. The adjusted HRs of arrhythmia, CAD, and CHF decreased with an increase in the follow-up durations. The study cohort with a ≤3-year follow-up revealed the highest risk of arrhythmia (adjusted HR = 2.59, 95% CI = 1.86–3.60) and CAD (adjusted HR = 1.33, 95% CI = 1.00–1.76). Figure 1A and 1B show that, compared with the comparison cohort, the study cohort exhibited a significantly higher cumulative incidence of arrhythmia (Figure 1A) and CAD (Figure 1B), as assessed using a log-rank test (P < 0.001).
TABLE 5.
Trends of Cardiovascular Event Risks by Stratified Follow-Up Years
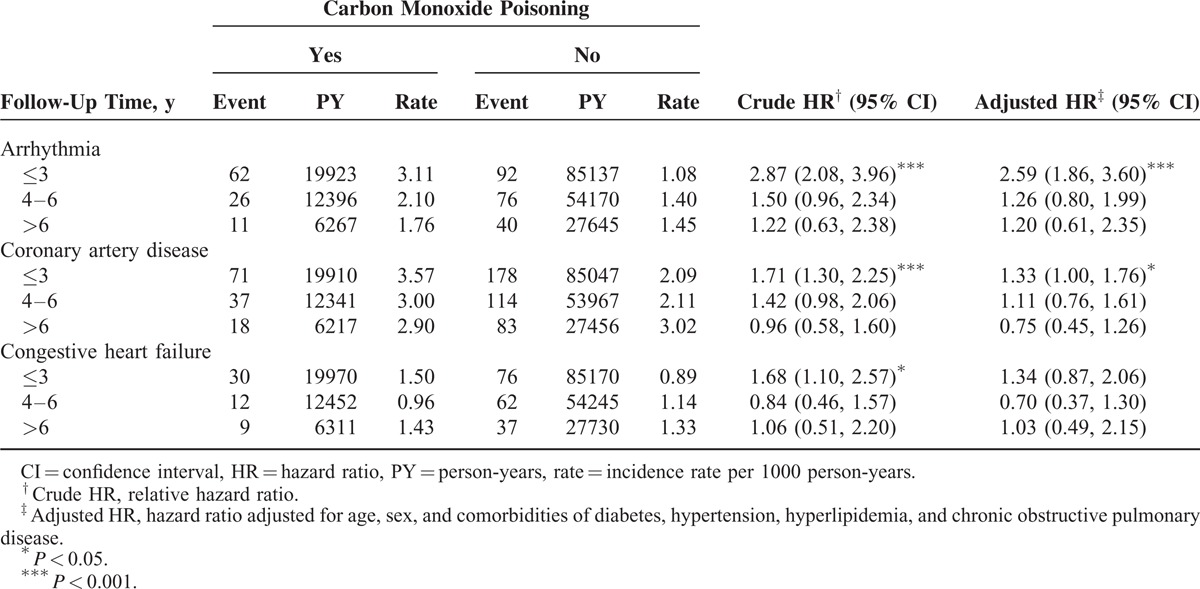
FIGURE 1.
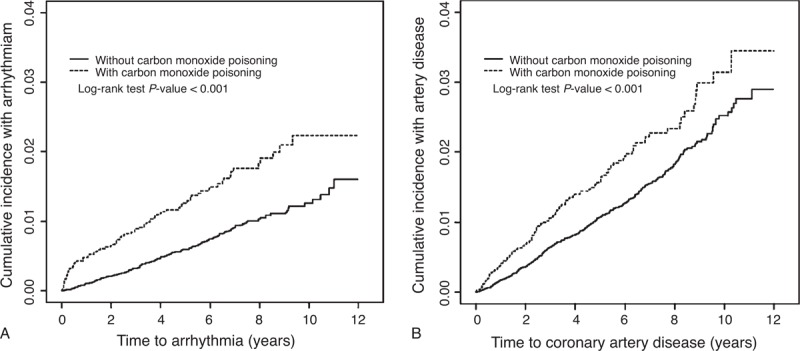
Cumulative incidence of (A) arrhythmia and (B) coronary artery disease compared between with and without carbon monoxide poisoning.
The results of multivariable Cox proportional hazard regression models for the risk of related variables contributing to arrhythmia, CAD, and CHF are shown in Table S1, http://links.lww.com/MD/A226. The risk factors contributing to arrhythmia included increasing age (in 1-y bands; adjusted HR = 1.05, 95% CI = 1.05–1.06), hypertension (adjusted HR = 2.86, 95% CI = 2.13–3.83), and COPD (adjusted HR = 2.09, 95% CI = 1.46–2.98). Older age (adjusted HR = 1.06, 95% CI = 1.05–1.06), men (adjusted HR = 1.51, 95% CI = 1.25–1.81), diabetes (adjusted HR = 1.57, 95% CI = 1.26–1.95), hypertension (adjusted HR = 4.23, 95% CI = 3.36–5.34), and hyperlipidemia (adjusted HR = 4.12, 95% CI = 3.30–5.15) were associated with an increased risk of CAD. Hypertension (adjusted HR = 3.28, 95% CI = 2.34–4.58), diabetes (adjusted HR = 2.29, 95% CI = 1.68–3.12), and COPD (adjusted HR = 1.85, 95% CI = 1.26–2.71) were also associated with an increased risk of CHF.
DISCUSSION
To our knowledge, this is the first nationwide, population-based cohort study to demonstrate the effects of CO poisoning on the risk of subsequent development of CVD. The results indicate an association of CO poisoning with higher overall crude risk of subsequent development of arrhythmia and CAD. The data remained statistically significant for arrhythmia after adjustment of confounders such as sex, age, and comorbidity. By contrast, no significant correlation was determined between CO poisoning and the overall risk of subsequent development of CHF.
Previous studies exploring the effects of CO poisoning on cardiovascular dysfunction have been limited to small-scale surveys or isolated case reports that focused on the transient toxic effects of CO poisoning. CO-related cardiovascular dysfunction includes angina, myocardial infarction, arrhythmia, left-ventricular dysfunction, transient myocardial stunning, cardiogenic shock, and sudden death.12,13 It has been established that the cardiotoxicity of CO is caused by dual effect, including tissue hypoxia and direct effects on the myocardium.14 CO connects with myoglobin and interferes with its function as an oxygen reservoir and consequent oxygen release. When CO binds to cytochrome oxidase in the mitochondria, the electron-transport chain and consequent ATP production are interrupted, which result in anaerobic respiration and formation of lactate and free radicals. Other effects, such as relaxation of vessel smooth muscles, inflammation, and thrombotic tendency, contribute to further injury.7,15,16 Moreover, the compensatory tachyarrhythmia because of systemic hypoxia in the early stages of CO poisoning increases the oxygen demand and accelerates CO diffusion, which further exacerbates the hypoxic injury of the myocardium.5 Furthermore, other studies have demonstrated that myocardial injury independently predicts the short-term poor outcome in patients with severe CO poisoning and the long-term mortality in those with moderate–to-severe CO poisoning.9,17 Therefore, emergency physicians should always identify features of myocardial injury by screening with electrocardiogram, cardiac markers, and other laboratory (eg, B-type natriuretic peptide) or imaging (echocardiography or coronography) tests.7 In recent years, dysregulated microRNAs (miRNAs) have been confirmed as a crucial factor in the development of all CVDs. They bind to complementary sequences within the mRNAs of target genes and, therefore, fine-tune the gene expression.18 Another study demonstrated the specific miRNAs secreted from normal human bronchial epithelial cells after brief exposure to CO.19 It deserves further investigations to elucidate the actual role of miRNAs and their potential diagnostic and/or prognostic applications in CO-related cardiac dysfunctions.
The findings of our study indicate a correlation between CO poisoning and increased subsequent risk of developing arrhythmia and CAD, which, although possibly including both immediate or transient toxic effects and chronic effects, can be beneficially informative for clinical decision makers and physicians. It is worth noting that the main types of arrhythmia documented in our study were paroxysmal tachycardia (24%), ventricular fibrillation or flutter (15%), and paroxysmal supraventricular tachycardia (7%), which is consistent with the findings that tachyarrhythmia is more prevalent in CO-poisoned patients considering a compensatory response to hypoxia and cardiac dysfunction.8,10,11,15,20 In addition, the incidence rate of arrhythmia was marginally higher in men than in women in the CO poisoning and comparison cohorts, which is consistent with the results of prior studies.21–25 However, despite the fact that the elderly exhibited the highest incidence rate of arrhythmia in both of the study and comparison cohorts, the youngest age group patients and those without coexisting comorbidity revealed a considerable risk after CO exposure, which implies that these groups of patients deserve special attention in the clinical process of care. Akdemir et al26 recently reported a 42-year-old female patient with CO poisoning who experienced an episode of atrial fibrillation with rapid ventricular response; her heart rhythm spontaneously returned to normal sinus rhythm after a 3-hour treatment with normobaric oxygen. They concluded that the short-term arrhythmia resulted from a conduction disorder secondary to tissue hypoxia. Cetin et al27 reported a 17-year-old CO-poisoned female patient with initial supraventricular tachycardia rhythm that returned to normal rhythm after the administration of diltiazem with no recurrence within 1 year of follow-up. Autonomic dysfunction caused by systemic hypoxia may trigger a possible concealed accessory or a slow pathway responsible for the supraventricular tachycardia rhythm. All these reports focused on the immediate and transient toxic effects on the heart.28 However, even with adequate treatment, delayed cardiac sequelae may occur. Grant and Clay15 reported 2 cases of compromised cardiac output with pulmonary edema and delayed dysrhythmia in 3-year-old twin girls several days after exposure to a high level of CO. Meanwhile, Yanir et al29 reported cases of CO poisoning in 2 young adults complicated by delayed tachyarrhythmia and cardiogenic shock; however, these patients exhibited favorable recovery of their neurologic and metabolic statuses. Reportedly, the production of reactive oxygen species in the hyperoxygenation process was believed to inflict re-oxygenation injury.20
Limited studies have reported a correlation between CO poisoning and CAD.30 Dziewierz et al31 reported a 36-year-old male patient who presented with ST-elevation myocardial infarction (STEMI) after CO poisoning; subsequently, a coronary angiogram revealed an acute occlusion of the distal left anterior descending coronary artery. Kim et al32 reported a case of STEMI secondary to CO poisoning with total occlusion of the posterior descending branch of the right coronary artery and a large thrombus burden. Studies have proposed that a combination of CO to fibrinogen-bound heme enhances the coagulation process, thereby promoting a thrombotic tendency, whereas myocardial stunning and an unbalanced oxygen demand and supply may reveal the underlying CAD.30–32 The most severe type of coronary thrombosis secondary to CO poisoning was recently reported by Dragelyte et al,14 wherein an 83-year-old female patient developed severe myocardial infarction complicated with myocardial rupture, cardiac tamponade, and collapse. It is well-known that the conventional risk factors such as smoking, diabetes, dyslipidemia, hypertension, and family history are crucial for the development of CAD, but they do not fully explain the pathophysiologic process of atherothrombosis, wherein CO-related thrombotic tendency, inflammation, and oxidative stress could play a role.33 In our study, the higher incidence rate of CAD was seen predominantly in men, the elderly, and those with comorbidity, which is consistent with prior reports.25 However, after eliminating the potential confounding effect by regression analysis, young age (≤49 years), female, and absence of comorbidity remained independent predictors of CAD in CO-poisoned patients. A possible explanation is that the presence and accumulation of complex comorbid factors or hidden confounders diluted the effects of CO.
Our study demonstrated the minor effects of CO poisoning on the development of CHF. Jung et al34 retrospectively surveyed 626 CO-poisoned patients and reported the development of cardiomyopathy in 3.04% of all patients; they suggested that myocardial stunning subjected to catecholamine surge, which induces a negative inotropic effect on the ventricular myocytes, was the possible underlying pathogenesis. Kalay et al1 prospectively evaluated 20 patients with CO poisoning and reported a correlation between the decrease in left ventricular ejection fraction and the carboxyhemoglobin levels and exposure duration. Satran et al10 described 2 major clinically favorable factors for the development of CO-induced cardiomyopathy: younger patients (average age: 43 years) with less risk factors were more prone to the development of global left-ventricular dysfunction, whereas among older patients (average age: 64 years) with more risk factors, most presented with regional wall abnormalities. Swank et al11 concluded the myocardial depression effect secondary to CO exposure resolved quickly and thus did not require long-term treatment. We hypothesize that CO-induced cardiomyopathy may be transient and mild and may quickly resolve under adequate oxygen therapy without any long-term recurrence or may be even overlooked during the entire clinical process, which may, in turn, contribute to the minor role of CO in the development of heart failure.
Furthermore, the interaction analysis performed in the present study revealed that conventional risk factors, which were grouped under comorbidity, play a predominant role in the development of the aforementioned 3 major CVDs. To assess the relative weight of CO and each conventional risk factor in detail, we performed Cox regression analysis and the result indicated that CO plays an essential role, but not a central role in the development of arrhythmia, whereas hypertension and hyperlipidemia rather than CO poisoning predominate in the development of CAD. However, despite the fact observed in the analysis, we should not overlook the occult influences of CO on cardiovascular system in clinical practice.
Hampson and Hauff35 reviewed 1505 consecutive CO-poisoned patients treated at a single institution and reported that severe metabolic acidosis and the need for endotracheal intubation exhibited the strongest association with short-term mortality. In our study, because of the unavailability of laboratory data for metabolic acidosis or the fact that ICD coding for acidosis was frequently missed in clinical practice, acute respiratory failure (ICD-9-CM code 518.81) diagnosed within the first 3 days of hospitalization was considered as the indicator to distinguish between the high- and the low-severity groups and to analyze the effects of CO poisoning under varying degrees of severity. The results revealed that the patients with severe CO poisoning exhibited the highest risk of developing all the 3 major CVDs, with adequate statistical significance.
Next, we performed a time-trend analysis to evaluate the risk of the 3 aforementioned CVDs by stratifying the follow-up period into 3 segments: (≤3, 4–6, and >6 years). The CO-poisoned patients exhibited a significantly higher risk of developing arrhythmia and CAD during the 3-year follow-up period. We believe that the development of cardiac toxicity-related immediate, transient, and self-limited heart dysfunction during the short period after CO exposure, known as “acute effect,” may have contributed to these results. By contrast, the effects of CO were nonsignificant during the mid- (4–6 years) and long-term (>6 years) follow-up periods, which implied that CO-related long-term cardiovascular sequelae were rare or that these effects were diluted by other uncontrolled confounders or complex comorbid factors that appeared with time. In addition, the average follow-up duration for arrhythmia was 4.60 ± 3.13 years for the study cohort and 4.98 ± 3.01 years for the comparison cohort. The mean follow-up period for CAD was 4.59 years for the study cohort and 4.97 years for the comparison cohort. From this point of view we suppose that CO plays a role in facilitating subsequent arrhythmia and CAD morbidity.
We used a sample derived from the NHIRD, which covers 99% of residents in Taiwan and offers comprehensive diagnostic medical information. The major strength of our study was the relatively large sample size, which served as an appropriate representative of the population and yielded more stable results. In addition, the datasets were identified based on diagnostic codes, thereby avoiding selection bias. Moreover, the claim database facilitated accurate and clear observations for each event occurrence during the study period.
Our study has several limitations. First, the study was designed as a retrospective cohort survey that does not adequately explain the causal relationships between the independent and the dependent variables. Second, the ICD-9-CM coding used for disease definition and sample extraction avoided most of the selection bias because the coding was meticulously reviewed by the physicians and administration personnel of the respective medical institutions; however, miscoding, misclassification, over or under coding may still exist, which cannot be verified or validated. Third, the NHIRD lacks information regarding family history, educational background, socioeconomic status, body mass index, cigarette smoking habits, severity of comorbidities, laboratory data, left-ventricular function, hyperbaric oxygen therapy protocols, and drug prescription details, which are crucial factors that influence cardiovascular morbidity, and we could not adjust for these confounders. Fourth, because of unavailability of laboratory data (such as level of carboxyhemoglobin, lactate or cardiac necrosis markers) and certain clinical information (such as state of consciousness, presence of neurologic deficits or echocardiogram findings) that are crucial for determination of poison's severity, the only indicator we considered, acute respiratory failure, with which to draw the conclusion might be risky. Fifth, because the NHIRD claims data records are available only from 2000, we could not determine whether the enrolled patients suffered from certain CVDs or experienced CO-poisoning events before the index date. Sixth, all the patients were tracked until the occurrence of the disease event, loss to follow-up, death, or until the end of 2011; therefore, the samples included in the later stages may not be sufficient for long-term morbidity trend analysis. Finally, our study design was based on the entire population of Taiwan. Considering the differences in geographical and epidemiological distributions, our results may not necessarily be applicable to other countries.
In conclusion, our research demonstrates a possible correlation between CO poisoning and the subsequent risk of developing arrhythmia. Although the exact mechanisms that underlie this association remain unclear, this observation could be informative for clinicians in managing CO-poisoned patients with increased alertness and aggressiveness by identifying evidences of myocardial damage and related heart dysfunction. However, further research is required to clarify the long-term effects of CO poisoning on the cardiovascular system and determine the relative predictors.
Footnotes
Abbreviations: CAD = coronary artery disease, CHF = congestive heart failure, CI = confidence interval, CO = carbon monoxide, COPD = chronic obstructive pulmonary disease, CVDs = cardiovascular diseases, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, NHIRD = National Health Insurance Research Database.
Authorship Contributions: Conception and design: All authors.
Administrative support: C-HK.
Collection and assembly of data: All authors.
Data analysis and interpretation: All authors.
Manuscript writing: All authors.
Final approval of manuscript: All authors.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212–113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103–2325-B-039 -006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and Health, and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TDU-B-212–124–002, Taiwan). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
All authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Kalay N, Ozdogru I, Cetinkaya Y, et al. Cardiovascular effects of carbon monoxide poisoning. Am J Cardiol 2007; 99:322–324. [DOI] [PubMed] [Google Scholar]
- 2.Ruth-Sahd LA, Zulkosky K, Fetter ME. Carbon monoxide poisoning: case studies and review. Dimens Crit Care Nurs 2011; 30:303–314. [DOI] [PubMed] [Google Scholar]
- 3.Choi IS. Carbon monoxide poisoning: systemic manifestations and complications. J Korean Med Sci 2001; 16:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Unintentional non-fire-related carbon monoxide exposures-United States, 2001–2003. MMWR 2005; 54:36–39. [PubMed] [Google Scholar]
- 5.Chiew AL, Buckley NA. Carbon monoxide poisoning in the 21st century. Crit Care 2014; 18:221. [Google Scholar]
- 6.Santulli G. Epidemiology of cardiovascular disease in the 21st century: updated numbers and updated facts. JCvD 2013; 1:1–2. [Google Scholar]
- 7.Szponar J, Kolodziej M, Majewska M, et al. Myocardial injury in the course of carbon monoxide poisoning. Przegl Lek 2012; 69:528–534. [PubMed] [Google Scholar]
- 8.Teksam O, Gumus P, Bayrakci B, et al. Acute cardiac effects of carbon monoxide poisoning in children. Eur J Emerg Med 2010; 17:192–196. [DOI] [PubMed] [Google Scholar]
- 9.Henry CR, Satran D, Lindgren B, et al. Myocardial injury and long-term mortality following moderate to severe carbon monoxide poisoning. JAMA 2006; 295:398–402. [DOI] [PubMed] [Google Scholar]
- 10.Satran D, Henry CR, Adkinson C, et al. Cardiovascular manifestations of moderate to severe carbon monoxide poisoning. J Am Coll Cardiol 2005; 45:1513–1516. [DOI] [PubMed] [Google Scholar]
- 11.Swank G, Jain AC, Morise AP, et al. Carbon monoxide poisoning: a case report of reversible cardiormyopathy. W V Med J 2004; 100:228–231. [PubMed] [Google Scholar]
- 12.Garg J, Krishnamoorthy P, Palaniswamy C, et al. Cardiovascular abnormalities in carbon monoxide poisoning. Am J Ther 2014. [DOI] [PubMed] [Google Scholar]
- 13.Lippi G, Rastelli G, Meschi T, et al. Pathophysiology, clinics, diagnosis and treatment of heart involvement in carbon monoxide poisoning. Clin Biochem 2012; 45:1278–1285. [DOI] [PubMed] [Google Scholar]
- 14.Dragelyte G, Plenta J, Chmieliauskas S, et al. Myocardial rupture following carbon monoxide poisoning. Case Rep Crit Care 2014; 2014:281701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant M, Clay B. Accidental carbon monoxide poisoning with severe cardiorespiratory compromise in 2 children. Am J Crit Care 2002; 11:128–131. [PubMed] [Google Scholar]
- 16.Ryoo SM, Sohn CH, Kim HJ, et al. Intracardiac thrombus formation induced by carbon monoxide poisoning. Hum Exp Toxicol 2013; 32:1193–1196. [DOI] [PubMed] [Google Scholar]
- 17.Kao HK, Lien TC, Kou YR, et al. Assessment of myocardial injury in the emergency department independently predicts the short-term poor outcome in patients with severe carbon monoxide poisoning receiving mechanical ventilation and hyperbaric oxygen therapy. Pulm Pharmacol Ther 2009; 22:473–477. [DOI] [PubMed] [Google Scholar]
- 18.Wronska A, Kurkowska-Jastrzebska I, Santulli G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol 2015; 213:60–83. [DOI] [PubMed] [Google Scholar]
- 19.Purushottam SN, Carolea L, Rongman C, Junfeng S, James HS, Anthony FS. The Secretion Of miRNA From Normal Human Bronchial Epithelial Cells: Effects Of Endotoxin, Nitric Oxide Or Carbon Monoxide. C32. ASTHMA: PRE-CLINICAL STUDIES: American Thoracic Society; 2014:A4220-A4220. [Google Scholar]
- 20.Gandini C, Castoldi AF, Candura SM, et al. Carbon monoxide cardiotoxicity. J Toxicol Clin Toxicol 2001; 39:35–44. [DOI] [PubMed] [Google Scholar]
- 21.Bernal O, Moro C. Cardiac arrhythmias in women. Rev Esp Cardiol 2006; 59:609–618. [PubMed] [Google Scholar]
- 22.Lampert R, McPherson CA, Clancy JF, et al. Gender differences in ventricular arrhythmia recurrence in patients with coronary artery disease and implantable cardioverter-defibrillators. J Am Coll Cardiol 2004; 43:2293–2299. [DOI] [PubMed] [Google Scholar]
- 23.Villareal RP, Woodruff AL, Massumi A. Gender and cardiac arrhythmias-. Texas Heart Inst J 2001; 28:265–275. [PMC free article] [PubMed] [Google Scholar]
- 24.Wolbrette D, Naccarelli G, Curtis A, et al. Gender differences in arrhythmias. Clin Cardiol 2002; 25:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics-2014 update: a report from the American Heart Association. Circulation 2014; 129:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akdemir HU, Gungorer B, Caliskan F, et al. Atrial fibrillation related to carbon monoxide poisoning in a female patient. Am J Emerg Med 2014. [DOI] [PubMed] [Google Scholar]
- 27.Cetin M, Ornek E, Murat SN, et al. A case of carbon monoxide poisoning presenting with supraventricular tachycardia. Intern Med 2011; 50:2607–2609. [DOI] [PubMed] [Google Scholar]
- 28.Carnevali R, Omboni E, Rossati M, et al. Electrocardiographic changes in acute carbon monoxide poisoning. Minerva Medica 1987; 78:175–178. [PubMed] [Google Scholar]
- 29.Yanir Y, Shupak A, Abramovich A, et al. Cardiogenic shock complicating acute carbon monoxide poisoning despite neurologic and metabolic recovery. Ann Emerg Med 2002; 40:420–424. [DOI] [PubMed] [Google Scholar]
- 30.Isik T, Tanboga IH, Guvenc TS, et al. ST-elevation myocardial infarction after acute carbon monoxide poisoning. Anadolu Kardiyol Derg 2012; 12:278–279.author reply 279. [DOI] [PubMed] [Google Scholar]
- 31.Dziewierz A, Ciszowski K, Gawlikowski T, et al. Primary angioplasty in patient with ST-segment elevation myocardial infarction in the setting of intentional carbon monoxide poisoning. J Emerg Med 2013; 45:831–834. [DOI] [PubMed] [Google Scholar]
- 32.Kim S, Lim JH, Kim Y, et al. A case of acute carbon monoxide poisoning resulting in an ST elevation myocardial infarction. Korean Circ J 2012; 42:133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santulli G. Coronary heart disease risk factors and mortality. JAMA 2012; 307:1137–1138. [DOI] [PubMed] [Google Scholar]
- 34.Jung YS, Lee JS, Min YG, et al. Carbon monoxide-induced cardiomyopathy. Circ JV 78 2014; 1437–1444. [DOI] [PubMed] [Google Scholar]
- 35.Hampson NB, Hauff NM. Risk factors for short-term mortality from carbon monoxide poisoning treated with hyperbaric oxygen. Crit Care Med 2008; 36:2523–2527. [DOI] [PubMed] [Google Scholar]


