Abstract
Monotherapy with telbivudine or adefovir can affect estimated the glomerular filtration rate (eGFR). However, only a few studies have assessed changes in eGFR in patients who have chronic hepatitis B (CHB) and are receiving nucleos(t)ide analogue (NA) combination therapy. In our study, we aimed to evaluate the effects of long-term NA combination therapy on eGFR in Chinese CHB patients.
This retrospective study included 195 CHB patients. Patient subgroups included those treated with lamivudine plus adefovir (n = 73), telbivudine plus adefovir (n = 51), and entecavir plus adefovir (n = 35); untreated patients (n = 36) served as a control group.
After an average follow-up duration of 24 months with combination therapy, analysis of changes in eGFR from baseline values, calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) formulas, showed decrease by11.08 and 18.34 mL/min (P < .001), respectively, in the lamivudine plus adefovir group; decrease by 3.73 and 10.04 mL/min (P = .012), respectively, in the entecavir plus adefovir group; and increase by 0.91 and 2.12 mL/min (P = .46), respectively, in the telbivudine plus adefovir group. The eGFR in the telbivudine plus adefovir group was similar to that for the untreated group. The eGFR decreases due to adefovir therapy could be rescued by adding telbivudine, and the eGFR increase due to telbivudine could be compromised by adding adefovir.
Adefovir in combination with lamivudine or entecavir therapy was significantly associated with decreased eGFR, but telbivudine could rescue the eGFR decrease that results from adefovir treatment.
INTRODUCTION
Currently, nucleos(t)ide analogues (NAs) are an important class of antiviral drugs that have changed the treatment paradigm for, and prognosis of, chronic hepatitis B (CHB).1 However, a few patients do not achieve virological response, even when treated with potent NA drugs. One-year virological response rates of 36%, 13%, 60%, and 67% have been reported for HBeAg-positive patients treated with lamivudine (LAM), adefovir (ADV), telbivudine (LdT), and entecavir (ETV), respectively.2 The virological response is important because a high residual viral level after the first 6–12 months of therapy has been demonstrated to be associated with an increased risk of antiviral resistance.3 NA combination therapy is optional according to the roadmap for hepatitis B virus (HBV) treatment.
The development of viral resistance is an issue faced with NA therapy.4,5 Five-year cumulative resistance rates of 67%, 18%, 1.2%,2 and 10.6%6 have been reported in patients treated with LAM, ADV, ETV, and LdT, respectively. Patients who develop virological breakthrough as a result of resistance mutations frequently experience acute exacerbations of disease and more rapid progression to acute liver failure, liver transplant, and increased risk of hepatocellular carcinoma (HCC) and death.7–10 Increasing evidence suggests that combination therapy has the potential to become an attractive therapeutic option in the management of these patients for the purpose of reducing viral resistance and virological breakthrough.11–16
For many years in China, the addition of ADV, which does not share cross-resistance with the other drugs, was the only therapeutic option for CHB patients with viral resistance or a poor virological response. Long-term use of ADV has been associated with dose-dependent renal toxicity in animal and human studies.17 Renal toxicity is caused by proximal tubule injury18–20 leading to abnormal phosphorus absorption21–24 and resulting in increased serum creatinine and renal dysfunction.25–26 To prevent disease progression, early identification of renal dysfunction is important, especially in patients who already have renal impairment at baseline.27
In clinical practice, serum creatinine alone has been used as an indicator to monitor renal impairment. However, serum creatinine levels are affected by age, sex, and weight, and cannot provide an accurate estimation of changes in renal function.28,29 The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) guideline suggests the use of the Chronic Kidney Disease Epidemiology Collaboration(CKD-EPI) equation to calculate estimated glomerular filtration rates (eGFRs) in adults.30 In addition, the Modification of Diet in Renal Disease (MDRD) formula is also widely used for the assessment of eGFR.31,32
Recently, some studies using eGFR to assess changes in renal function during NA therapy in CHB patients reported that eGFR decreases with ADV treatment27,33 and increases with LdT treatment.6,34–36 Nevertheless, only a few clinical studies have been performed to assess renal function using eGFR in CHB patients treated (due to viral resistance or a poor virological response to monotherapy) with different NA combination therapies; these studies had inconsistent results.37,38 Our aim was to evaluate changes in renal function on the basis of eGFR levels in Chinese CHB patients receiving long-term NA combination therapies, that is, LAM plus ADV, LdT plus ADV, and ETV plus ADV.
METHODS
Patients
We performed a retrospective cohort study of 195 patients (aged 18–70 years) who had chronic HBV infection and were followed up at our hospitals from January 2009 to December 2013. These patients, who showed resistance to NAs or did not have a virological response, were categorized into 3 combination therapy groups: 73 in the LAM plus ADV group (56 with resistance to LAM, 2 did not have avirological response to LAM, 12 did not have avirological response to ADV, 3 with resistance to ADV), 51 in the LdT plus ADV group (38with resistance to LdT, 1 did have the virological response to LdT, 8 did not have the virological response to ADV, 4 with resistance to ADV), and 35 in the ETV plus ADV group (6 with resistance to ETV, 24 did not have the virological response to ADV, 5 with resistance to ADV). All treated patients received an average of 24 months of monotherapy and achieved virological response within 1 year after combination treatment. The control group consisted of 36 untreated patients, who were in the immune-tolerant phase.
The major inclusion criteria included HBV monoinfection with detectable HBsAg at screening and for at least 6 months prior to the treatment. NA-treated patients also had a history of not achieving a virological response after 48 weeks of treatment or had been proven to have viral resistance. Exclusion criteria included eGFR ≤60 mL/min or serum creatinine >1.5 mg/dL at screening, combined with hypertension, diabetes, or chronic renal insufficiency.
The study was in compliance with the Helsinki Declaration and was approved by the Medical Ethics Committee of Fudan University Huashan Hospital and Shanghai Public Health Clinical Center. All the enrolled patients gave their written informed consent.
Study Design
Patients who needed combination antiviral therapy were given LAM plus ADV, LdT plus ADV, or ETV plus ADV. All the patients were followed up once every 3 months. Qualitative tests for albuminuria, HBV-DNA load, serum creatinine, serum creatine kinase (CK), and aminotransferase (ALT) and quantitative analyses of HBsAg, HBeAg, and HBeAb were performed. Using the data for serum creatinine levels, the eGFR was calculated using the CKD-EPI equation39 and MDRD equation.31,32 All patients were divided into 3 groups: unimpaired (eGFR ≥90 mL/min), mildly impaired (60 ≤eGFR <90 mL/min), and moderately impaired (30 ≤eGFR <60 mL/min) groups.40 The end-point was defined by the termination of either the treatment or the study.
Statistical Methods
All data were analyzed with the Stata 10.0 software (Stata Corporation, College Station, TX). A 2-sided P value <0.05 was considered statistically significant. Categorical variables were expressed as number (%), and continuous variables were expressed as the median (range). Using a Student t test or a Wilcoxon test, continuous variables were compared before and after treatment. Data for multiple groups were processed using the one-way analysis of variance or Kruskal–Wallis test; categorical variables were processed using the χ2 test or Fisher exact test. The logistic regression model was used to estimate univariate and multivariate odds ratios (ORs) of various factors related to changes in eGFR.
RESULTS
Baseline Characteristics
The combination therapy groups were matched by their baseline characteristics (Table 1) for age (±2 years), sex, duration of observation, HBV DNA load, baseline serum creatinine, baseline eGFR, and baseline eGFR classification.
TABLE 1.
Baseline characteristics of chronic hepatitis B patients∗
Only the Telbivudine Plus Adefovir Group did not Show a Significant Decrease in eGFR
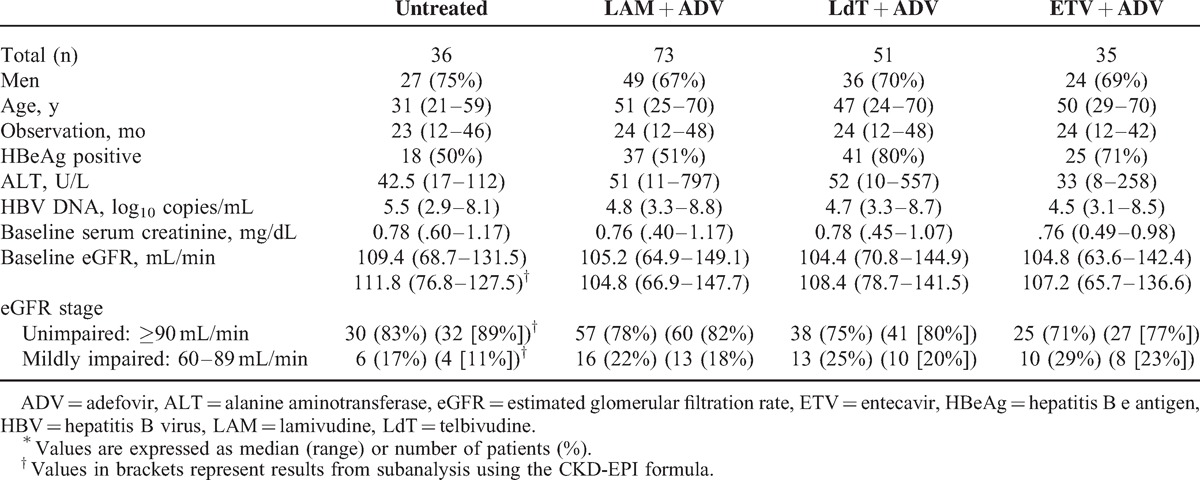
At baseline, serum creatinine was within the normal range in all patients. However, 23% (MDRD) and 18% (CKD-EPI) of the patients had eGFR <90 mL/min, respectively, suggesting that these patients already had mild renal impairment (Table 1). The eGFR values calculated by the 2 formulas were similar (x2= 1.57, P = .21). Therefore, we used only the MDRD formula for the calculation of eGFR values in the subsequent analyses. During treatment, eGFR decreased steadily in the ETV plus ADV and the LAM plus ADV treatment groups, and the annual eGFR decrease from baseline was 9.46 mL/min in the first year, 20.31 mL/min in the second year, and 26.64 mL/min in the third year (all P < .001 vs baseline) for the LAM plus ADV group. In the ETV plus ADV group, the annual eGFR decrease from baseline was 3.58 mL/min in the first year (P = .04), 13.85 mL/min in the second year (P = .005), and 24.92 mL/min in the third year (P < .001; Fig. 1). However, in the LdT plus ADV group, eGFR did not show a significant difference compared with the baseline; the annual eGFR increase from baseline was 1.86 mL/min in the first year (P = .24), 1.71 mL/min in the second year (P = .41), and 1.6 mL/min in the third year (p = .66). The eGFR of 10 patients, that is, 9 patients treated with LAM plus ADV therapy and 1 with ETV plus ADV therapy, progressed to a classification of moderately impaired (30 ≤eGFR <60 mL/min). However, only 3 patients (2%) treated with LAM plus ADV therapy had albuminuria and an incrementof ≥0.5 mg/dL in serum creatinine. These results indicate that serum creatinine is less sensitive than eGFR for evaluating renal function.
FIGURE 1.
Changes in renal function-associated indicators during follow-up in patients treated with different NA combination therapies. (A) Serum creatinine; (B) eGFR calculated by the MDRD equation; (C) eGFR calculated by the CKD-EPI equation.
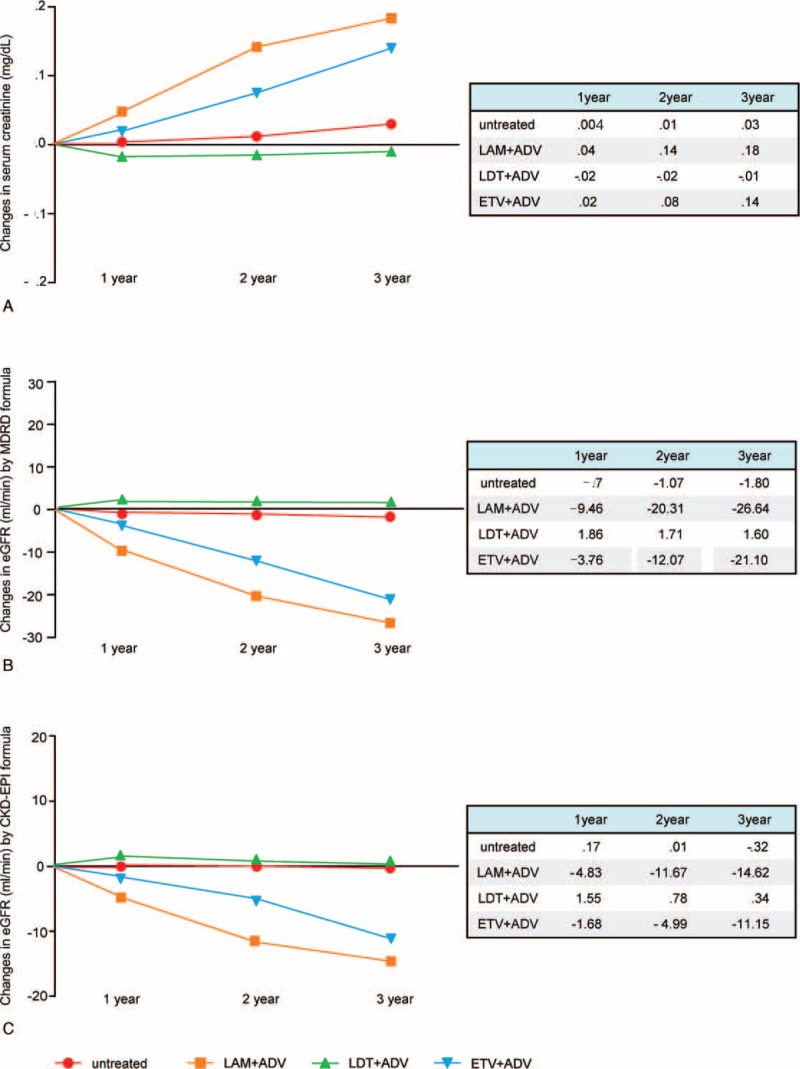
After an average follow-up of 24 months, the change in eGFR from baseline was −18.34 mL/min (P < .001), 2.12 mL/min (P = .46), −10.04 mL/min (P = .01), and −1.28 mL/min (P = .66) in the LAM plus ADV, LdT plus ADV, ETV plus ADV, and untreated groups, respectively (Fig. 2). Compared with the findings for the other groups, eGFR was significantly lower in the LAM plus ADV group (P < .001 vs untreated; P < .001 vs LdT plus ADV; P = .01 vs ETV plus ADV) and in the ETV plus ADV group (P = .03 vs untreated; P = .004 vs LdT plus ADV). The eGFR did not show significant change only in the LdT plus ADV group (P = .38 vs untreated).
FIGURE 2.
Changes in the eGFR of patients after each NA combination therapy.
Changes in eGFR in Patients With Mild Renal Impairment at Baseline
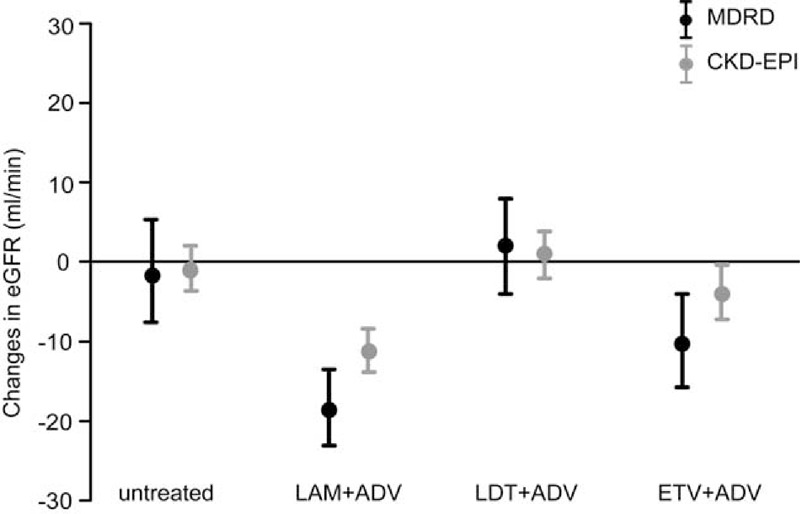
After LdT plus ADV treatment, eGFR increased by 12.52 mL/min (P = .005) in patients with renal impairment at baseline, whereas it increased by 1.39 mL/min (P = .69) in patients with normal renal function, which is a significant difference (P = .03; Fig. 3). Consistent with this reduction, the proportion of the patients who had mild renal impairment decreased from 27% at baseline to11%.
FIGURE 3.
Changes in the eGFR at week 104 in patients with and without mild renal impairment at baseline. eGFRcalculated by (A) the MDRD equation and (B) the CKD-EPI equation.∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
Conversely, after LAM plus ADV treatment, eGFR decreased by 10.81 mL/min (P = .009) and 20.46 mL/min (P < .001) in patients with or without renal impairment at baseline, respectively, which is a significant difference (P = .04) (Fig. 3). Accordingly, the proportion of the patients who had mild renal impairment increased from 35% at baseline to 62%.
Predictors Associated With Significant eGFR Decrease
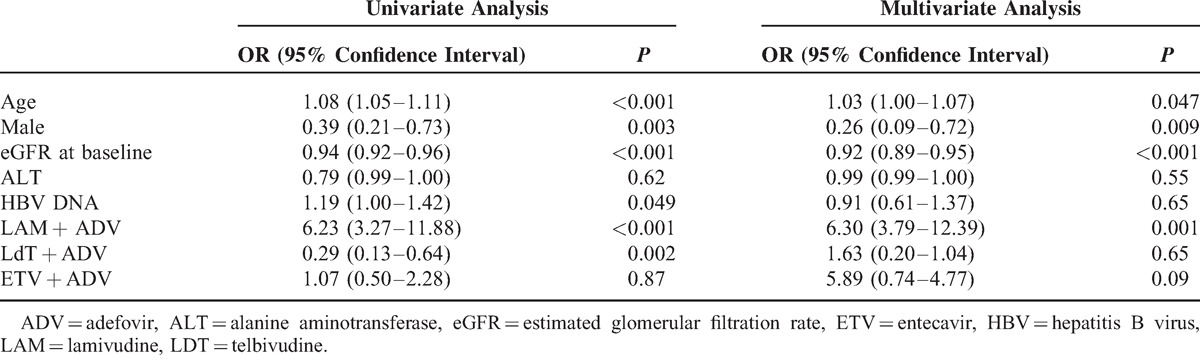
Univariate and multivariate predictors and ORs for an eGFR decrease of >20% are displayed in Table 2. Multivariate analysis showed that age (OR, 1.03; P = .047), sex (OR, .26; P = .009), eGFR at baseline (OR, .92; P < .001), and treatment with LAM plus ADV (OR, 6.30; P = .001) were all independent predictors for >20% decrease in eGFR.
TABLE 2.
Predictors for >20% eGFR Decrease From Baseline
Decrease in eGFR after Adefovir Monotherapy Could be Rescued by Adding On Telbivudine
To further analyze the impact of NAs on eGFR, we specifically analyzed the patients who received ADV monotherapy before combination therapy. After ADV monotherapy, eGFR had decreased by 20.02 mL/min, 21.26 mL/min, and 19.40 mL/min (all P < .001), prior to the patients receiving LAM plus ADV, LdT plus ADV, or ETV plus ADV, respectively. After an average 24-month follow-up with combination therapy, the eGFR further decreased by 10.41 mL/min (P < .001 vs untreated) in the LAM plus ADV group and by 9.03 mL/min (P < .001 vs untreated) in the ETV plus ADV group. However, eGFR increased by 15.99 mL/min in the LdT plus ADV group, and the final eGFR for these patients was similar to that for the untreated group (Fig. 4a).
FIGURE 4.
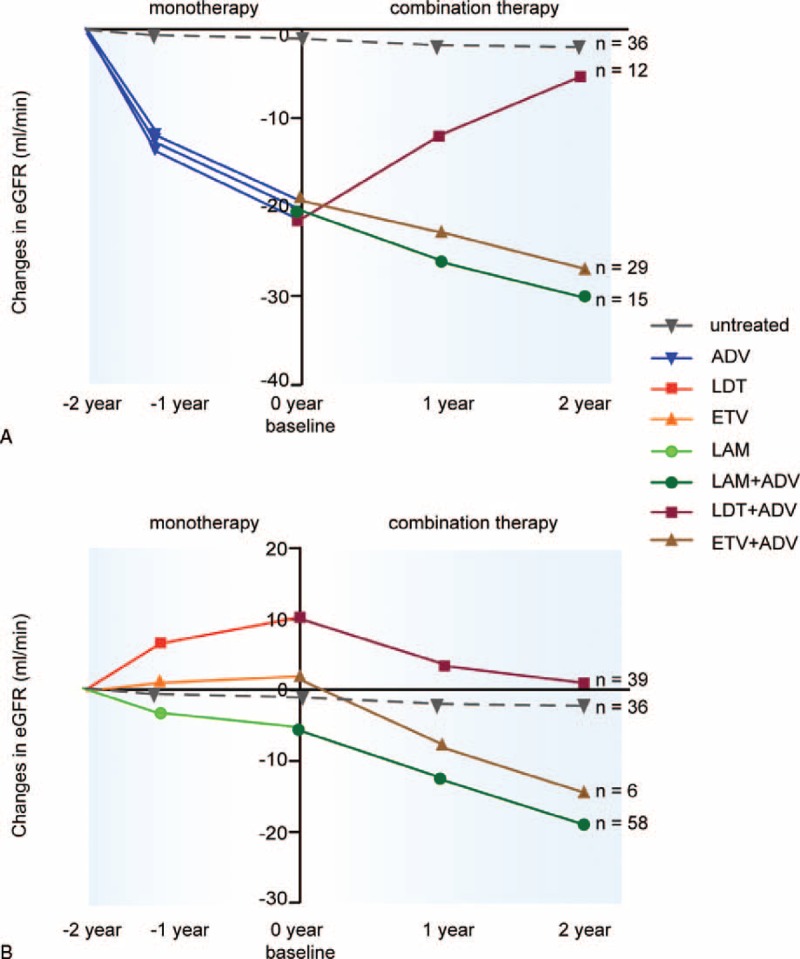
Changes in eGFR (MDRD) in patients who shifted from monotherapy to combination therapy. (A) Patients who did not achievevirological response after receiving adefovir treatment. (B) Patients who showed resistance to NAs.
Increase in eGFR After Telbivudine Monotherapy Could be Compromised by Adding On Adefovir
To further analyze the impact of NAs on eGFR, we specifically analyzed the patients who received LAM, LdT, or ETV monotherapy before ADV was added on. During monotherapy, the eGFR decreased by 4.83 mL/min in the patients receiving LAM, increased by 2.04 mL/min in the patients receiving ETV, and increased by 10.07 mL/min in the patients receiving LdT. After an average 24-month follow-up with combination therapy, the eGFR further decreased by 15.01 mL/min (P < .001 vs untreated) in the LAM plus ADV group and decreased by16.35 mL/min (P < .001 vs untreated) for the ETV plus ADV group. However, the eGFR only decreased by 9.49 mL/min in the LdT plus ADV group, and the final eGFR was not significantly different from that for the untreated group (Fig. 4b).
DISCUSSION
To our knowledge, this is the first cohort clinical study that compared the effects of three different NA combination therapies on eGFR in Chinese CHB patients. Our results indicated that LAM plus ADV and ETV plus ADV therapy resulted in decreased eGFR, whereas LdT plus ADV therapy did not significantly influence eGFR and the eGFR values were similar to those for the untreated group.
NAs reduce viral replication by inhibition of HBV polymerase and are therefore not able to completely eradicate HBV. The majority of CHB patients will require long-term treatment. Therefore, the safety profiles of these drugs are of paramount importance, especially the effect on renal function. We used eGFR instead of creatinine to evaluate renal function because a given serum creatinine level may represent different levels of eGFR and renal function, depending on the patient's weight, age, and race; therefore, such definition of renal impairment as used in clinical studies may underestimate the incidence of clinically significant renal impairment. Chen et al found that approximately 41.9% of 15540 patients aged 35 to 74 years in China with eGFR <90 mL/min had normal serum creatinine.41 Similarly, 23% (as per the MDRD equation) and 18% (as per the CKD-EPI equation) of the patients in our study, whose serum creatinine levels were within the normal range at baseline, already had mild renal impairment. Therefore, eGFR is more accurate and effective for estimating renal function.17,32
In our study, we found that LdT plus ADV therapy did not significantly influence eGFR after long-term therapy and could even reverse renal dysfunction for the patients with eGFR under the normal range at the baseline, which is consistent with previous studies.37,42 This may be correlated with the significantly increased eGFR in the group treated with LdT.34,36 Conversely, for the patients treated with LAM plus ADV, the decrease in eGFR was significant, which is consistent with the findings described by Vigano et al.38 Lee et al37 used a linear mixed-effects regression model that indicated LAM plus ADV therapy could significantly improve eGFR, which contradicted the results of this study. They reported only a moderate effect of LAM on improving eGFR in combination therapy with ADV (estimated at 0.15 ± 0.04)37 and they incorporated baseline eGFR as a fixed effect in their analysis because the study groups were not matched by baseline eGFR. In addition, the inclusion criteria used by Lee et al differed from those used in this study. It is possible that differences in the study design and statistical analysis could explain the discrepancy between these results. It will be important to follow-up these results in a future study to completely understand the impact of LAM on eGFR.
Considering that eGFR decreases are found in patients receiving ADV and LAM monotherapies, it is possible that LAM could potentiate the decrease in eGFR caused by ADV in combined therapy. The multivariate analysis for a >20% eGFR decrease from baseline showed that age, baseline eGFR, and the use of LAM plus ADV were independent predictors. Therefore, LAM plus ADV is not recommended for use in the elderly with chronic HBV infection and renal impairment.
We also found that 23% of CHB patients had mild renal impairment at baseline. After long-term therapy, LdT plus ADV could significantly improve eGFR in this group of patients, and even reverse renal dysfunction, which is consistent with the findings described by Lee et al.37 Additionally, when CHB patients were treated with LAM plus ADV, the decrease in eGFR was more significant in patients with normal renal function than in the patients with mild renal impairment at baseline. This may explain why only a few patients with mild renal impairment at baseline progressed to moderate renal impairment after this therapy and why serum creatinine rarely increased >0.5 mg/mL after long-term treatment.
In our previous study, we found prolonged that LdT monotherapy resulted in improved eGFR, whereas ADV monotherapy was associated with decreased eGFR,36 which was consistent with studies performed by other groups.27,33–35 In this combination therapy study, LdT plus ADV did not significantly influence eGFR, which suggests that the renoprotective effects of LdT could overcome the nephrotoxicity caused by ADV. It is believed that ADV may be directly toxic to the proximal tubular cells, which is related to the drug's activity against the human ϒ-mitochondrial DNA (mtDNA).17 The proximal tubular dysfunction may decrease glomerular filtration rates by tubuloglomerular feedback. Depletion of mtDNA from the renal tubular epithelium may also contribute to decreased glomerular filtration rates.26 As shown in Fig. 3A, LdT could rescue the eGFR decrease due to adefovir during the average 24-month combination therapy, resulting in eGFR levels similar to those in the untreated group, and this effect showed a continually increasing trend. However, the mechanism by which telbivudine improves eGFR is still not clear. As a possible mechanism, Chan et al43 suggested that LdT could increase blood flow, and thereby improve tubular dysfunction. Liang et al suggested LdT could decrease serum angiotensin-converting enzyme levels, inhibiting the renin-angiotensin aldosterone regulatory system, with known downstream effects on systemic vasoconstriction, renal sodium, and renal fluid retention.44
The limitations of this study are the small sample size and relatively short observation duration. Thus, clinical trials with large sample size and long-term follow-up are needed to confirm our findings. Because of the unavailability of tenofovir, we could not evaluate the changes in eGFR in the CHB patients treated with combination therapy including tenofovir, and further studies are needed to determine these changes. As this is a clinical retrospective study, a patient bias also exists because all treated patients received the average 24-month monotherapy before combination therapy.
CONCLUSION
In summary, the results of this clinical study suggest that LAM plus ADV therapy and ETV plus ADV therapy resulted in decreased eGFR, whereas LdT plus ADV therapy did not significantly influence eGFR and could even reverse eGFR in the patients with mild renal dysfunction. For patients with renal dysfunction, adding on LdT might be a good choice for combination therapy.
Acknowledgments
We thank Dr Xiaoqin Wang (Fudan University) for her helpful suggestions on statistical analysis during the study.
Footnotes
Abbreviations: ADV = adefovir, ALT = alanine aminotransferase, BMI = body mass index, CHB = chronic hepatitis B, CK = creatine kinase, CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration, eGFR = estimated glomerular filtration rate, ETV = entecavir, HBeAb = hepatitis B e antibody, HBeAg = hepatitis B e antigen, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HR = hazard ratio, KDIGO = Kidney Disease, Improving Global Outcomes, LAM = lamivudine, LdT = telbivudine, MDRD = modification of diet in renal disease, mtDNA = mitochondrial DNA, NA = nucleos(t)ide analogue, ULN = upper limit of the normal range.
XQ and JW contributed equally to the work.
This work was financially supported by the National Natural Science Foundation of China (81201277, 81271833), Doctoral Fund of Ministry of Education of China (20120071120099, 20130071110055), Major Research and Development Project of Innovative Drugs (2012ZX09303004–001), Major Science and Technology Special Project of China (2012ZX10002007–001–002, 2013ZX10002001), 973 Project (2012CB519001), the fund of Health and Family Planning Commission (201302010), and Shanghai Pujiang Program (12PJ1401600).
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Yuen M-F, Lai C-L. Treatment of chronic hepatitis B: evolution over two decades. J Gastroenterol Hepatol 2011; 26 Suppl 1:138–181. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012; 57:167–185. [DOI] [PubMed] [Google Scholar]
- 3.Zoulim F. Hepatitis B virus resistance to antiviral drugs: where are we going? Liver Int 2011; 31 Suppl 1:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hongthanakorn C, Chotiyaputta W, Oberhelman K, et al. Virological breakthrough and resistance in patients with chronic hepatitis B receiving nucleos(t)ide analogues in clinical practice. Hepatology 2011; 53:1854–1863. [DOI] [PubMed] [Google Scholar]
- 5.Zoulim F, Locarnini S. Hepatitis B virus resistance to nucleos(t)ide analogues. Gastroenterology 2009; 137:1593–1608. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Thongsawat S, Gane EJ, et al. Efficacy and safety of continuous 4-year telbivudine treatment in patients with chronic hepatitis B. J Viral Hepat 2013; 20:e37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 2004; 351:1521–1531. [DOI] [PubMed] [Google Scholar]
- 8.Lok AS, Lai CL, Leung N, et al. Long-term safety of lamivudine treatment in patients with chronic hepatitis B. Gastroenterology 2003; 125:1714–1722. [DOI] [PubMed] [Google Scholar]
- 9.Nafa S, Ahmed S, Tavan D, et al. Early detection of viral resistance by determination of hepatitis B virus polymerase mutations in patients treated by lamivudine for chronic hepatitis B. Hepatology 2000; 32:1078–1088. [DOI] [PubMed] [Google Scholar]
- 10.Di Marco V, Marzano A, Lampertico P, et al. Clinical outcome of HBeAg-negative chronic hepatitis B in relation to virological response to lamivudine. Hepatology 2004; 40:883–891. [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Cheong J, Lee D, et al. Adefovir-based combination therapy with entecavir or lamivudine for patients with entecavir-refractory chronic hepatitis B. J Med Virol 2012; 84:18–25. [DOI] [PubMed] [Google Scholar]
- 12.Chen EQ, Wang LC, Lei J, et al. Meta-analysis: adefovirdipivoxil in combination with lamivudine in patients with lamivudine-resistant hepatitis B virus. Virol J 2009; 6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chae HB, Kim MJ, Seo EG, et al. High efficacy of adefovir and entecavir combination therapy in patients with nucleoside-refractory hepatitis B. Korean J Hepatol 2012; 18:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen EQ, Zhou TY, Bai L, et al. Lamivudine plus adefovir or telbivudine plus adefovir for chronic hepatitis B patients with suboptimal response to adefovir. Antivir Ther 2012; 17:973–979. [DOI] [PubMed] [Google Scholar]
- 15.Seto WK, Liu K, Fung J, et al. Outcome of lamivudine-resistant chronic hepatitis B after up to 5 years of combination therapy with adefovir. AntivirTher 2012; 17:1255–1262. [DOI] [PubMed] [Google Scholar]
- 16.Shin JW, Jung SW, Park BR, et al. HBV DNA level at 24 weeks is the best predictor of virological response to adefovir add-on therapy in patients with lamivudine resistance. Antivir Ther 2012; 17:387–394. [DOI] [PubMed] [Google Scholar]
- 17.Van Rompay KK, Durand-Gasselin L, Brignolo LL, et al. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob Agents Chemother 2008; 52:3144–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Prada FJ, Prados AM, Tugores A, et al. Acute renal failure and proximal renal tubular dysfunction in a patient with acquired immunodeficiency syndrome treated with tenofovir. Nefrologia 2006; 26:626–630. [PubMed] [Google Scholar]
- 19.Malik A, Abraham P, Malik N. Acute renal failure and Fanconi syndrome in an AIDS patient on tenofovir treatment-case report and review of literature. J Infect 2005; 51:E61–65. [DOI] [PubMed] [Google Scholar]
- 20.Peyrière H, Reynes J, Rouanet I, et al. Renal tubular dysfunction associated with tenofovir therapy: report of 7 cases. J Acquir Immune Defic Syndr 2004; 35:269–273. [DOI] [PubMed] [Google Scholar]
- 21.Quimby D, Brito MO. Fanconi syndrome associated with use of tenofovir in HIV-infected patients: a case report and review of the literature. AIDS Read 2005; 15:357–364. [PubMed] [Google Scholar]
- 22.Rifkin BS, Perazella MA. Tenofovir-associated nephron toxicity: Fanconi syndrome and renal failure. Am J Med 2004; 117:282–284. [DOI] [PubMed] [Google Scholar]
- 23.Woodward CL, Hall AM, Williams IG, et al. Tenofovir-associated renal and bone toxicity. HIV Med 2009; 10:482–487. [DOI] [PubMed] [Google Scholar]
- 24.Agarwala R, Mohan S, Herlitz LC, et al. The case: 41-year-old HIV patient with proteinuria and progressive renal dysfunction. Tenofovir toxicity. Kidney Int 2010; 77:475–476. [DOI] [PubMed] [Google Scholar]
- 25.Herlitz LC, Mohan S, Stokes MB, et al. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int 2010; 78:1171–1177. [DOI] [PubMed] [Google Scholar]
- 26.Izzedine H, Launay-Vacher V, Deray G. Antiviral drug-induced nephrotoxicity. Am J Kidney Dis 2005; 45:804–817. [DOI] [PubMed] [Google Scholar]
- 27.Mauss S, Berger F, Filmann N, et al. Effect of HBV polymerase inhibitors on renal function in patients with chronic hepatitis B. J Hepatol 2011; 55:1235–1240. [DOI] [PubMed] [Google Scholar]
- 28.Duncan L, Heathcote J, Djurdjev O, et al. Screening for renal disease using serum creatinine: Who are we missing? Nephrol Dial Transplant 2001; 16:1042–1046. [DOI] [PubMed] [Google Scholar]
- 29.Swedko PJ, Clark HD, Paramsothy K, et al. Serum creatinine is an inadequate screening test for renal failure in elderly patients. Arch Intern Med 2003; 163:356–360. [DOI] [PubMed] [Google Scholar]
- 30.Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150. [Google Scholar]
- 31.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130:461–470. [DOI] [PubMed] [Google Scholar]
- 32.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007; 53:766–772. [DOI] [PubMed] [Google Scholar]
- 33.Ha NB, Ha NB, Garcia RT, et al. Renal dysfunction in chronic hepatitis B patients treated with adefovirdipivoxil. Hepatology 2009; 50:727–734. [DOI] [PubMed] [Google Scholar]
- 34.Piratvisuth T, Komolmit P, Tanwandee T, et al. 52-week efficacy and safety of telbivudine with conditional tenofovir intensification at week 24 in HBeAg-positive chronic hepatitis B. PLoS One 2013; 8:e54279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gane EJ, Deray G, Liaw YF, et al. Telbivudine improves renal function in patients with chronic hepatitis B. Gastroenterology 2014; 146:138–146. [DOI] [PubMed] [Google Scholar]
- 36.Qi X, Wang J, Mao R, et al. Impact of nucleos(t)ide analogues on the estimated glomerular filtration rate in patients with chronic hepatitis B: A prospective cohort study in China. J Viral Hepat 2015; 22:46–54. [DOI] [PubMed] [Google Scholar]
- 37.Lee M, Oh S, Lee HJ, et al. Telbivudine protects renalfunction in patients with chronic hepatitis B infection in conjunction with adefovir-based combination therapy. J Viral Hepat 2014; 21:873–881. [DOI] [PubMed] [Google Scholar]
- 38.Vigano M, Lampertico P, Lavarone M, et al. High risk of renal impairment during long-term adefovir and lamivudine combination therapy in patients with lamivudine-resistant chronic hepatitis B. J Hepatol 2009; 50:S338–S339. [Google Scholar]
- 39.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003; 139:137–147. [DOI] [PubMed] [Google Scholar]
- 41.Chen J, Wildman RP, Gu D, et al. Prevalence of decreased kidney function in Chinese adults aged 35 to 74 years. Kidney Int 2005; 68:2837–2845. [DOI] [PubMed] [Google Scholar]
- 42.Sun J, Xie Q, Tan D, et al. The 104-week efficacy and safety of telbivudine-based optimization strategy in chronic hepatitis B patients: a randomized, controlled Study. Hepatology 2014; 59:1283–1292. [DOI] [PubMed] [Google Scholar]
- 43.Chan HL, Chen YC, Gane EJ, et al. Randomized clinical trial: efficacy and safety of telbivudine and lamivudine in treatment-naive patients with HBV-related decompensated cirrhosis. J Viral Hepat 2012; 19:732–743. [DOI] [PubMed] [Google Scholar]
- 44.Liang KH, Chen YC, Hsu CW, et al. Decrease of serum angiotensin converting enzyme levels upon telbivudine treatment for chronic hepatitis B virus infection and negative correlations between the enzyme levels and estimated glumerular filtration rates. Hepat Mon 2014; 14:e15074. [DOI] [PMC free article] [PubMed] [Google Scholar]


