Abstract
Amyloid load, as measured by florbetapir positron emission tomography (PET) standardized uptake value ratio (SUVr), has high specificity in the diagnosis of Alzheimer disease (AD). As the posterior cingulate cortex (PCC) represents densely amyloid-affected regions early in AD, we hypothesized that amyloid load within the key hubs of the default mode networks (DMN) may result in local or distant interconnected gray matter (GM) volume atrophy, thereby affecting cognitive performance. Thirty AD patients with a clinical dementia rating sum of box score ≤2 were enrolled and underwent cognitive evaluation, 3-dimensional T1-weighted imaging and florbetapir PET. Volumes of interest (VOIs) included the hippocampus, lateral temporal region, and key hubs of the DMN [anterior cingulate cortex (ACC), PCC, posterior parietal, and precuneus]. The SUVr was calculated by florbetapir standard uptake value (SUV) within the T1-weighted image segmented GM VOIs divided by the cerebellar GM SUV. Our results suggested inverse correlations between ACC (ρ = −0.444, P = 0.016) and PCC SUVr (ρ = −0.443, P = 0.016) with PCC GM volume. In stepwise regression, the orientation scores were associated with PCC SUVr (β = 2.584, P = 0.02) and posterior parietal volume (β = −0.446, P = 0.04), whereas the word recall score was related to hippocampal volume (β = −0.391, P = 0.04). After removing the patients with a hippocampal VOI below the lowest tertile and adjusting for age, an inverse correlation was found between hippocampal volume and SUVr in the ACC (partial σ = −0.639, P = 0.002), precuneus (partial σ = −0.692, P = 0.002), and lateral temporal SUVr (partial σ = −0.604, P = 0.005). Our results suggest that amyloid burden within the key DMN regions may contribute to local and distant GM atrophy, and that this may explain the cognitive scores.
INTRODUCTION
The International Working Group-2 criteria for Alzheimer disease (AD)1 recently redefined the clinical use of amyloid and glucose positron emission tomography (PET). Amyloid PET is considered to be a diagnostic marker that reflects in vivo pathology, whereas glucose PET is considered to represent a marker of downstream disease progression that reflects clinical severity. It has been hypothesized that with regards to amyloid-β in AD, self-aggregation of amyloid fibrils and accumulation of amyloid plaques would result in downstream network degeneration.2 The characteristic glucose PET patterns1 in AD are hypometabolism in the anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), precuneus, and temporo-parietal regions, collectively termed the default mode network (DMN).3 As cortical hubs within the DMN network represent early pathological targets in AD and are also highly functionally anchored, changes in cognitive scores may reflect local amyloid toxicity or related neuronal degeneration.
Using the amyloid tracer 11C Pittsburgh compound B, increased uptake in the PCC region can be detected early in AD, whereas uptake in the hippocampus and DMN key hubs has been associated with regional gray matter (GM) atrophy.4 In cognitively normal elderly subjects, Oh et al5 also observed that greater amyloid deposition in the DMN regions was associated with a greater loss in GM volume. Another study showed that regional florbetapir (AV-45) uptake using standardized uptake value ratio (SUVr) quantification achieved 96% sensitivity and 100% specificity in reflecting moderate-to-frequent neuritic plaques.6 Until recently, florbetapir PET has been considered to be a valid clinical tool in the diagnosis of AD.7 Rosenberg et al8 reported significant inverse correlations between verbal fluency score with precuneus florbetapir SUVr. However, it is not known whether cognitive relationships exist in other DMN hubs and how they are linked with changes in regional GM volume.
It is generally accepted that regional atrophy has a significant impact on the quantitative accuracy of PET imaging.9 Current morphometric analysis techniques in magnetic resonance imaging (MRI) allow for accurate brain tissue segmentation into GM, white matter or nonbrain regions.10 With an MRI-based coregistration algorithm, it is possible to quantify regional GM amyloid burden. The issue of appropriate head size adjustment has also been reported in the context of cortical structure changes in the elderly.11 Normalization of regional brain volumes by total intracerebral volume (ICV) [ie, the ratio between the volume of interest (VOI) and ICV] is often performed in disorders involving neurodegeneration,12 as well as adjustments for age or educational level when investigating regional volume data with cognitive scores. In addition, it is also known that changes in certain brain cortical structures such as hippocampal formation and middle and inferior temporal cortical structures are disproportional to other GM structures in AD.13 Use of ICV-normalized method might mask the changes related directly to pathology or in tissues with small volumes. In the literature, both raw regional volume14,15 and volume-to-ICV fraction16 have been used to investigate volumetric–cognitive relationships in AD.
As the PCC area represents a densely amyloid-affected region early in AD,17,18 we hypothesized that amyloid load within the key hubs of the DMN could result in local or distant connected GM volume atrophy, thereby affecting cognitive performance. In addition, since amyloid load often reaches a plateau at the dementia stage,19 the proof of concept of amyloid toxicity to morphologic or cognitive changes would theoretically be more feasible by selecting AD patients at a milder clinical stage. Based on image coregistration with GM-segmented high-resolution T1-weighted images, the florbetapir SUVr in 6 VOIs was calculated using the cerebellar GM as a reference region. For clinical correlation, we used the Alzheimer Disease Assessment Scale-cognitive subscale (ADAS-cog),20 a tool commonly used in patients with AD, to represent the cognitive performance. To examine whether volumetric measurements correlated with cognitive scores, both raw volume data expressed in cubic millimeters and ICV-normalized volume data were recorded.
METHODS
Inclusion and Exclusion Criteria
This was a single-center, prospective, and observational study. The patients were recruited from the Department of Neurology of Chang Gung Memorial Hospital from 2011 to 2014. All of the patients underwent comprehensive neurological and neuropsychological assessments with consensus rendered at a multidisciplinary conference.21,22 AD was diagnosed according to the International Working Group criteria for AD.23 Only patients with a clinical dementia rating (CDR)24 sum of box score ≤2.0 and positive reading results on florbetapir PET25 were selected. The rationale for choosing this CDR criterion was to avoid floor effects of cognitive performance and also to reach an earlier clinical stage prior to the plateau of amyloid deposition. All of the patients received acetylcholine esterase inhibitors from the time of diagnosis. The exclusion criteria were a history of clinical stroke, a modified Hachinski ischemic score > 4,26 and depression.
The hospital's Human Ethics Committee approved the study protocol, and all of the participants and their authorized caregivers provided written informed consent. AV-45 PET scan, cognitive testing, and MRI were all performed within a 4-week period.
MRI Acquisition and Volumetric Analysis
MRI was performed using a GE 3T Signa Excite scanner. Structural images were acquired for anatomic reference and to verify the clinical diagnosis using the following protocols T2-weighted, turbo spin-echo sequence with repetition time/echo time/number of averages of 4200/101.2 ms/2, 240 × 240 mm field of view, 320 × 224 matrix, and 5-mm axial slice thickness; and T1-weighted, inversion-recovery-prepared, 3-dimensional, spoiled, gradient-recalled acquisition in a steady-state sequence with repetition time/inversion time of 8600/450 ms, 240 × 240 mm field of view, and 1-mm slice thickness.
The preprocessing of T1 MRI involves removal of nonrelevant tissue, intensity normalization, and automatic Talairach transformation and segmentation. We used the Automated Anatomic Labeling atlas27 for regional labeling and volumetric calculation. In addition to the DMN cortical regions (posterior parietal, precuneus, ACC, and PCC), we also included the lateral temporal region (ie, superior, middle, and inferior lateral temporal regions) and hippocampal formation as VOIs (Figure 1).
FIGURE 1.
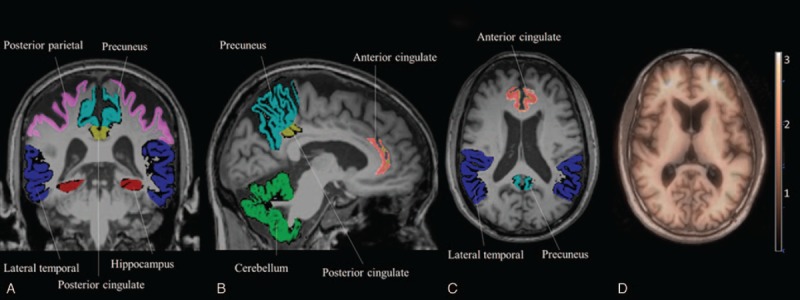
Map of the volumes of interest on brain MRI. (A) Coronal view. (B) Sagittal view. (C) Axial view. (D) Fusion of PET and MRI. MRI = magnetic resonance imaging, PET = positron emission tomography.
High intrasubject correlations between the right and left hemispheres were found among all 6 VOIs [from highest σ = 0.951 (P < 0.001) for the hippocampus to lowest σ = 0.824 (P < 0.001) for the ACC]. The average of left and right corresponding VOIs was used for statistical analysis.
AV-45 PET Acquisition and Analysis
AV-45 was synthesized at the cyclotron facility of Chang Gung Memorial Hospital. The PET acquisition protocol, optimal scanning time, and image reconstruction followed a previous report.25 In brief, helical computed tomography images were obtained for attenuation correction at 40 minutes. Each PET acquisition consisted of 3 5-minute dynamic frames obtained 50 minutes postinjection in 3-dimensional mode using a Biography molecular computed tomography PET/computed tomography system (Siemens Medical Solutions, Malvern, PA). Summed images were subsequently created for further analysis.
The PET images were first coregistered to the 3-dimensional T1 images by a nonlinear transformation using Statistical Parametric Mapping 8 software (Wellcome Trust Centre of Cognitive Neurology, University College London, London, UK). The 6 VOIs defined from the MRI were transferred to the coregistered PET images. A representative example of the fusion imaging between the MRI and PET images is shown in Figure 1D. The cerebellar GM represented the reference region (Figure 1B, green region). The SUV is related to injection dose and normalized to body weight. The SUVr was calculated by determining the ratios of SUV between the target VOI and the reference region.
Intrasubject Spearman correlations between the SUVr of the right and left hemispheres were high. The correlation coefficient for the lateral temporal region was σ = 0.738 (P < 0.001), precuneus σ = 0.935 (P < 0.001), ACC σ = 0.797 (P < 0.001), PCC σ = 0.864 (P < 0.001), posterior parietal σ = 0.940 (P < 0.001), and hippocampus σ = 0.818 (P < 0.001).
Neuropsychological Assessment
A trained neuropsychologist administered the tests. Cognitive function was assessed using the minimental state examination28 and the 11-item Chinese version of the ADAS-cog.20
Statistical Analysis
All values were expressed as mean ± standard deviation (SD). To assess the relationships between continuous variables including SUVr, cognitive test score, volumetric data, age, and education, Pearson correlation coefficients or Spearman rank correlation coefficients were calculated with a corresponding two-sided significance test at the 0.05 significance level. To assess the appropriateness of using parametric statistics for these analyses, we used the Kolmogorov–Smirnov test to examine the normality, and P values >0.05 indicated no significant deviations from normality. Both raw GM volume and ICV-normalized volume data (calculated by expressing the VOI as a proportion of the ICV) were recorded for further cognitive correlations. For volumetric measurements that correlated with regional SUVr or cognitive performance, age was entered as a covariate of no interest. Stepwise regression was carried out to determine the best predictors of cognitive score. Each model used SUVr or volumetric data, age, and education level (in years) as independent variables. All statistical analyses were conducted using the Statistical Package for Social Sciences software package (version 18 for Windows, SPSS Inc, Chicago, IL).
RESULTS
Clinical and Imaging Data
After the initial screening, 30 patients (15 males) with a mean age of 76.6 ± 8.0 years (range, 62–89 years) and an educational level of 8.6 ± 5.4 years (range, 0–18 years) completed the study. The total GM AV-45 SUVr was 1.29 ± 0.2 (range, 0.81–1.75), and in descending order was PCC 1.54 ± 0.4 (range, 0.99–2.71), precuneus 1.48 ± 0.3 (range, 0.84–2.21), ACC 1.47 ± 0.4 (range, 0.8–2.25), lateral temporal 1.34 ± 0.3 (range, 0.8–1.98), posterior parietal 1.27 ± 0.3 (range, 0.61–1.89), and hippocampus 0.002 ± 0.0003 (range, 0.001–0.0022).
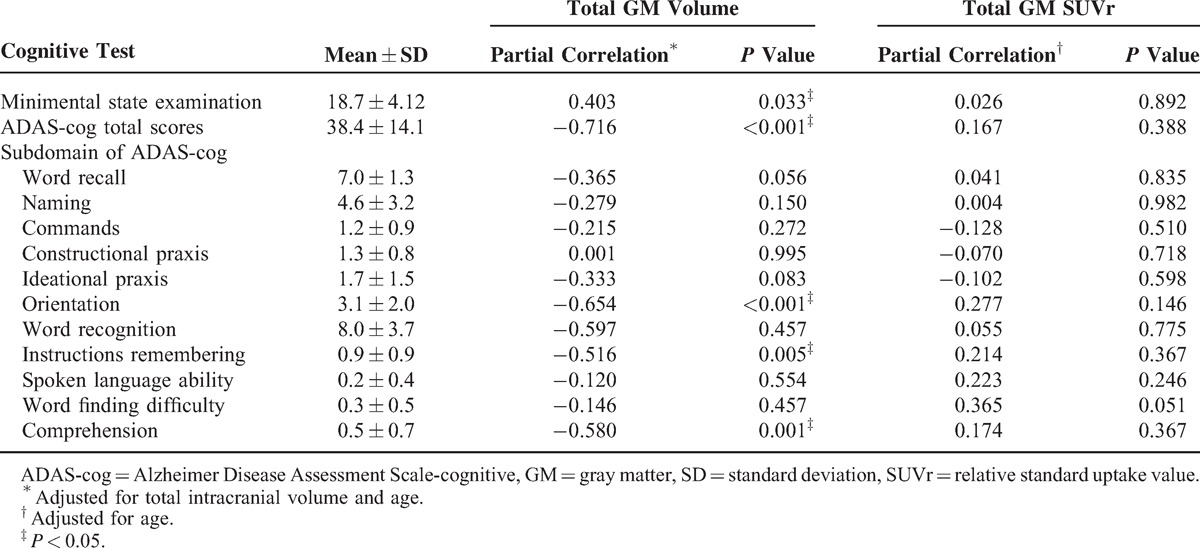
The ICV-normalized VOIs were ACC 0.326% ± 0.035%, PCC 0.163% ± 0.018%, posterior parietal 1.098% ± 0.122%, lateral temporal 2.639% ± 0.355%, precuneus 0.772% ± 0.121%, and hippocampus 0.182% ± 0.042%. There was a significant correlation between age and ADAS-cog score (σ = 0.367, P = 0.04). The total GM volume was correlated with minimental state examination score, ADAS-cog total, and subdomain scores including orientation, instruction remembering, and comprehension (Table 1). There were no significant correlations between total SUVr and the cognitive tests.
TABLE 1.
Clinical Data of the Alzheimer Disease Patients (n = 30)
Relationship Between Volumetric Measurements and SUVr Within the VOIs
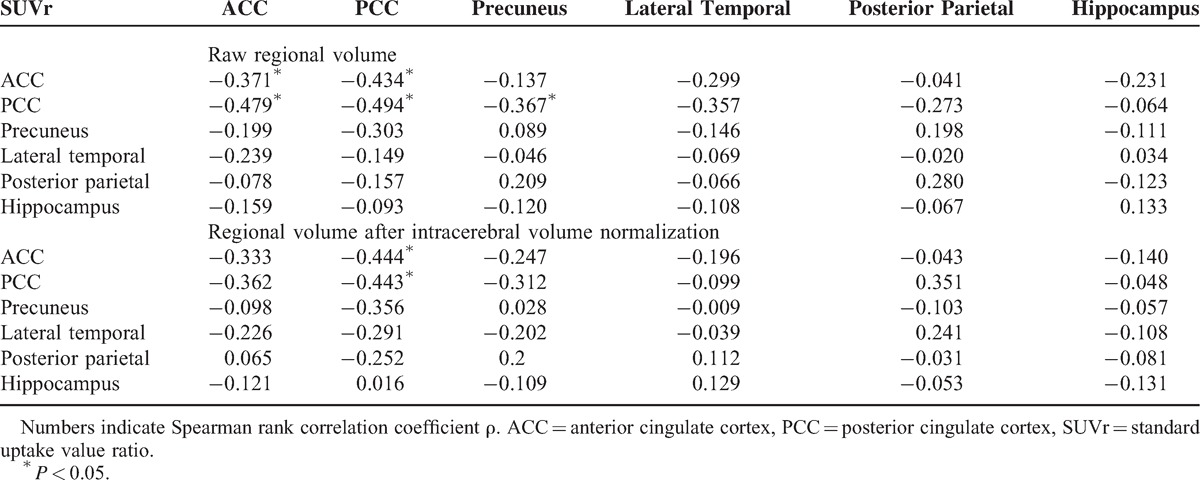
There were significant inverse correlations between ACC SUVr and raw volumes of the ACC (ρ = −0.371, P = 0.04) and PCC (ρ = −0.479, P = 0.01), and between PCC SUVr with the volume of the ACC (ρ = −0.434, P = 0.02), PCC (ρ = −0.494, P = 0.01), and precuneus (ρ = −0.367, P < 0.05) (Table 2). After ICV normalization, significant inverse correlations were found between ACC SUVr (ρ = −0.444, P = 0.016) or PCC SUVr (ρ = −0.443, P = 0.016) and PCC volume (Table 2).
TABLE 2.
Relationships Between Regional Cortical Volume and SUVr
Although there were no correlations between the ACC, precuneus or lateral temporal AV-45 SUVr, and hippocampal volume, the scatter plot showed a concave pattern (Figure 2A–C). We further removed the patients with a hippocampal VOI below the lowest tertile. After adjusting for age, there were significant linear relationships between the ICV-normalized hippocampal volume and SUVr in the ACC (partial σ = −0.639, R2 = 0.718, P = 0.002), precuneus (partial σ = −0.692, R2 = 0.3076, P = 0.001), and lateral temporal (partial σ = −0.604, R2 = 0.2837, P = 0.005) (Figure 2D–F).
FIGURE 2.
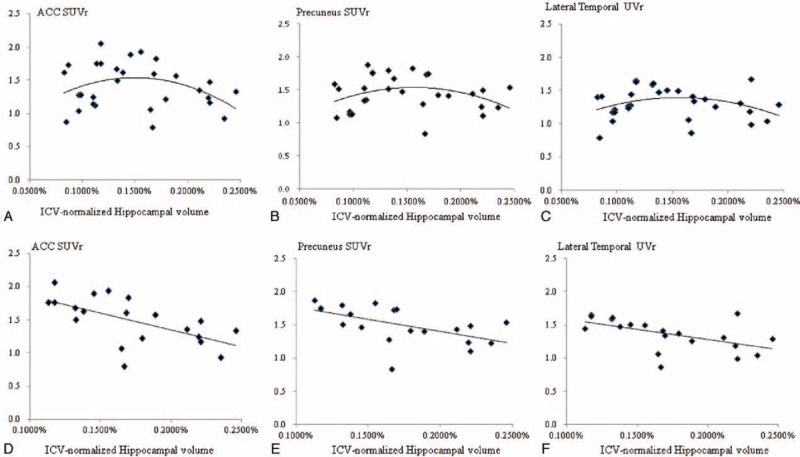
Scatter plot of the age-adjusted ICV normalized hippocampus volume with AV-45 SUVr) values in the ACC (A), precuneus (B), and lateral temporal region (C). Corresponding fitting curves in the 3 areas (D–F) in patients with a relatively preserved hippocampal volume. ACC = anterior cingulate cortex, ICV = intracerebral volume, SUVr = standard uptake value ratio.
Independent Role of PCC SUVr With Orientation Scores
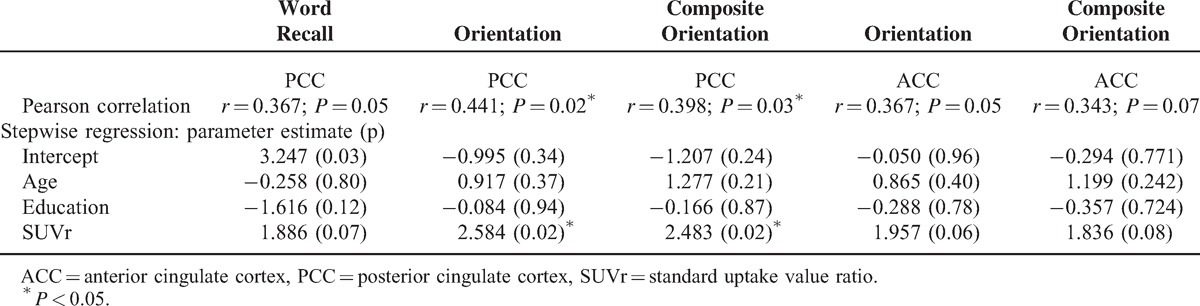
The only significant relationship between SUVr and the cognitive variables was found between the PCC SUVr and the orientation score (r = 0.441, P = 0.02) (Table 3). Therefore, we further calculated the composite orientation score to recheck the results. This was composed of the orientation box score of the CDR and the ADAS-cog orientation test score with each measure carrying the same weight. The PCC SUVr consistently showed an association with the composite orientation score (P < 0.05), and this association was still significant after stepwise regression analysis (Table 3). There was no significant association between PCC volume and orientation score or composite orientation score.
TABLE 3.
Correlation of Regional SUVr With Cognitive Test Performance
Independent Role of Volumetric Measurements With Orientation Scores
For the volumetric measurements that explained the orientation score, significant relationships were seen in the posterior parietal (ρ = −0.499, P = 0.01), precuneus (ρ = −0.486, P = 0.01), and ACC (ρ = −0.498, P = 0.01), whereas significance with composite orientation score was also seen in the volume of posterior parietal (ρ = −0.527, P = 0.01), precuneus (ρ = −0.506, P = 0.01), and ACC (ρ = −0.490, P = 0.01). This observation remained significant in the stepwise regression model. A larger posterior parietal volume was associated with better performance in the ADAS-cog (β = −0.498, R2 = 0.226, t = −2.934, P = 0.01), orientation score (β = −0.478, R2 = 0.259, t = −2.714, P = 0.01), and composite orientation score (β = −0.506, R2 = 0.312, t = −2.983, P = 0.01), and a larger ACC was associated with a better performance in orientation score (β = −0.470, R2 = 0.251, t = −2.652, P = 0.01) and composite orientation score (β = −0.472, R2 = 0.279, t = −2.718, P = 0.01).
After ICV normalization, significant relationships were seen between the orientation score and volume of posterior parietal (ρ = −0.425, P = 0.02) and precuneus (ρ = −0.463, P = 0.01), whereas significance with composite orientation score was also seen in the volume of posterior parietal (ρ = −0.478, P = 0.01) and precuneus (ρ = −0.504, P = 0.01). In the stepwise regression model, a larger posterior parietal volume was associated with better performance in orientation score (β = −0.446, R2 = 0.254, t = −2.240, P = 0.04) and composite orientation score (β = −0.494, R2 = 0.321, t = −2.599, P = 0.02).
Independent Role of Volumetric Measurements With Memory Score
The word recall subdomain score represented the memory domain in this study, and significance was found in the raw volume of hippocampus (β = −0.412, R2 = 0.186, t = −2.314, P = 0.029), PCC (β = −0.391, R2 = 0.171, t = −2.185, P = 0.038), and lateral temporal region (β = −0.378, R2 = 0.161, t = −2.101, P = 0.046). In the regression analysis, the independent relationships between the aforementioned VOIs with the word recall subdomain remained significant (β = −0.393 for hippocampus; β = −0.255 for PCC, and β = −0.235 for lateral temporal volume; P = 0.035).
After ICV normalization, significant relationships were found between word recall score and hippocampal volume (ρ = −0.368, P = 0.049) and lateral temporal volume (ρ = −0.392, P = 0.04). In the regression analysis, only hippocampal ICV-normalized volume showed a significant relationship with the word recall subdomain score (β = −0.391, R2 = 0.265, t = −2.158, P = 0.04).
DISCUSSION
Major Findings
This study investigated the associations between amyloid load and related volumetric measurements and cognitive test scores in mild stage AD, and there were 3 major findings. First, the results may suggest different clinical roles when considering AV-45 SUVr and volumetric data. Although the ACC and PCC AV-45 SUVr were both related to volume reductions in PCC, our results showed the influence of amyloid load on local and distant interconnected DMN cortical hubs. Second, significant correlations were seen between the PCC SUVr and the orientation scores that were not mediated by the PCC volume. Therefore, orientation scores may be mediated directly by local amyloid load or alternatively via reductions in GM in the interconnected posterior parietal regions. Third, the isolated amyloid load within the DMN was insufficient to explain the salient memory deficits, whereas relationships were only seen in the ICV-normalized hippocampal volumetric correlations. Therefore, although a direct influence of amyloid burden on cognitive deficit is still possible, our results suggest that a more sophisticated trajectory of cognitive deficits in mild stage AD may be predominantly modulated via degeneration of the interconnected DMN network.
Independent Role of PCC AV45 Burden on Orientation Scores
It is well known that the amyloid pathological process precedes both neuronal degeneration and clinical symptoms in AD.29 Although AV-45 PET imaging signals have been confirmed to reflect amyloid pathology,6,30 it is difficult in terms of temporal relationship to predict the onset of an individual cognitive deficit. PCC remains an early target of amyloid deposition whether in patients with AD17 or in cognitively normal subjects with a positive family history of AD.18 As our results showed that a higher AV-45 PCC SUVr was related to lower orientation composite score, the direct relationship between amyloid burden and cognitive outcome pointed to the importance of PCC. Specifically, although the orientation score is not a key presenting feature in diagnosing AD, it still represents one of the cognitive domains involved in the relatively early stage of AD.
The PCC, consisting of Brodmann areas 29, 30, 23, and 31 contains neurons that monitor eye movements and respond to sensory stimuli. The relationship between orientation scores and PCC SUVr in our study is consistent with a previous study in which a correlation between sensory events within the PCC was reported to explain the spatial orientation score.31 Although using amyloid load to assess the degree of cognitive impairment seems to be flawed in AD, our results were significant for patients with mild stage AD and after quantifying amyloid load within the corresponding GM, normalized to ICV in volumetric calculations, and in the statistical analysis which minimized possible confounding factors including educational level and age. The monitoring of amyloid-β toxicity for the effect on individual cognition performance apparently requires an earlier scanning window, whereas local AV-45 SUVr still provides clinical value in predicting cognitive performance. Our findings of regional amyloid burden within PCC regions with cognitive deficits add to the growing literature that AV-45 PET can help to quantify the pathophysiology in certain cognitive domains.8,32
Amyloid Load With Related Interconnected Volume Atrophy Targeting the ACC–PCC axis
Our results showed inverse correlations between ACC or PCC SUVr with PCC volume. The regional-specific relationships between AV-45 uptake and volumetric data in the PCC region may suggest concomitant amyloid toxicity that is directly linked to neuronal injury. Alternatively, it is also possible that the atrophic findings observed in our results encompass other pathological processes that are mechanistically linked to amyloid cascade. Meanwhile, the link between ACC amyloid load and a reduction in PCC volume here also implies that amyloid burden may disrupt connectivity in the functionally interconnected ACC–PCC axis.33 Further studies may be needed to verify our observations linking amyloid load with related network degeneration in AD, since high levels of ACC, PCC, or precuneus amyloid load have also been reported to be associated with very restricted regional atrophy.34 Nonetheless, a degenerative theme has also been reported by 2 other studies on elderly subjects.5,35
Hippocampus Volume Predicted Memory Scores
Episodic memory impairment is a salient feature of AD. Our results showed an inverse correlation between word recall subdomain score and ICV-normalized hippocampal volume.36,37 In contrast, none of the VOI SUVr showed clinical predictive value. In mild stage AD, the lack of relationships between amyloid-β burden and memory scores may indicate a protective cognitive reserve that may mask the pathological process of amyloid-β. In addition, it is also possible that the influence is limited in individuals with advanced hippocampal atrophy,19 since the inverse relationships between ACC, precuneus and lateral temporal AV-45 SUVr with hippocampal volume were only found in our AD patients with a relatively preserved hippocampal volume. Taken together with previous findings, we suggest that the relationship between amyloid burden and lower memory performance8,32 is more robust in healthy elderly subjects.8,32 In contrast, when AD patients are in the dementia phase, only regional precuneus SUVr is associated with verbal fluency scores.8
Quantification of AV-45 Densities by MRI-Based GM Segmentation
According to the literature, most amyloid-imaging data provide insignificant linkage to cognitive performance in AD. A reason for this may be related to the quantification methods. As AV-45 shows nonspecific binding in white matter,2,38 quantification of the amyloid-β burden focusing on the corresponding GM would theoretically be more straightforward. SUVr quantification in patients with GM atrophy based on an a priori anatomical template may overestimate the AV-45 retention density by sampling white matter signals. With regards to changes in GM volume, our GM segmentation-based SUVr quantification method was labor intensive and required manual rechecking to ensure correct coregistration in each subject. However, with individual MRI-based coregistration in SUVr quantification, the direct association of AV-45 SUVr with orientation score in this study reinforces the significance of this method.
STUDY LIMITATIONS AND CONCLUSION
The study limitations include a small sample size which may have led to the risk of type I and type II errors. As the analyses with specific cognitive assessments were exploratory, the reported P values in our results were unadjusted for multiple comparisons. The independent relationship between PCC SUVr and orientation performance was reported after careful statistical examination by correlational analysis, stepwise regression analysis, and rechecking the composite orientation score. In addition, there may have been selection bias in our population as they all only had mild stage AD. However, this was to avoid the oversaturation of AV-45 to directly explore the effect of amyloid burden on cognitive performance.
In conclusion, our study indicates that amyloid burden within the ACC and PCC regions may contribute to local or distant interconnected DMN cortical hub GM atrophy. Although the orientation scores were related to PCC amyloid load, changes in hippocampal volume could explain the salient memory score.
Acknowledgments
The authors express their gratitude to the patients who participated in this project and Miss Chen Ching for performing the cognitive test scores.
Footnotes
Abbreviations: ACC = anterior cingulate cortex, AD = Alzheimer disease, ADAS-cog = Alzheimer Disease Assessment Scale-cognitive subscale, CDR = clinical dementia rating, DMN = default mode network, GM = gray matter, ICV = intracerebral volume, MRI = magnetic resonance imaging, PCC = posterior cingulate cortex, PET = positron emission tomography, SD = standard deviation, SUVr = standardized uptake value ratio, VOI = volume of interest.
This study was supported in part by grants from Chang Gung Memorial Hospital (CMRPG 8B1001, CMRPG8C0571 and CMRPG8D0771) and National Research Program for Biopharmaceuticals (MOST 103-2325-B-182A-009).
All of the authors certify that the manuscript is a unique submission and is not being considered for publication by any other source in any medium. The study protocol was approved by the hospital's Institutional Review Committee on Human Research.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol 2014; 13:614–629. [DOI] [PubMed] [Google Scholar]
- 2.Weiner MW, Veitch DP, Aisen PS, et al. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement 2012; 8:S1–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greicius MD, Srivastava G, Reiss AL, et al. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 2004; 101:4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chetelat G, Villemagne VL, Bourgeat P, et al. Relationship between atrophy and beta-amyloid deposition in Alzheimer disease. Ann Neurol 2010; 67:317–324. [DOI] [PubMed] [Google Scholar]
- 5.Oh H, Habeck C, Madison C, et al. Covarying alterations in Abeta deposition, glucose metabolism, and gray matter volume in cognitively normal elderly. Hum Brain Mapp 2014; 35:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark CM, Pontecorvo MJ, Beach TG, et al. Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-beta plaques: a prospective cohort study. Lancet Neurol 2012; 11:669–678. [DOI] [PubMed] [Google Scholar]
- 7.Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. Alzheimers Dement 2013; 9:e-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg PB, Wong DF, Edell SL, et al. Cognition and amyloid load in Alzheimer disease imaged with florbetapir F 18(AV-45) positron emission tomography. Am J Geriatr Psychiatry 2013; 21:272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fazio F, Perani D. Importance of partial-volume correction in brain PET studies. J Nucl Med 2000; 41:1849–1850. [PubMed] [Google Scholar]
- 10.Ashburner J, Friston KJ. Voxel-based morphometry – the methods. Neuroimage 2000; 11:805–821. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg DL, Messer DF, Payne ME, et al. Aging, gender, and the elderly adult brain: an examination of analytical strategies. Neurobiol Aging 2008; 29:290–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voevodskaya O, Simmons A, Nordenskjold R, et al. The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer's disease. Front Aging Neurosci 2014; 6:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karow DS, McEvoy LK, Fennema-Notestine C, et al. Relative capability of MR imaging and FDG PET to depict changes associated with prodromal and early Alzheimer disease. Radiology 2010; 256:932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai RM, Leong JK, Dutt S, et al. The Chinese verbal learning test specifically assesses hippocampal state. Am J Alzheimers Dis Other Demen 2014; pii: 1533317514552667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng E, Chow N, Hwang KS, et al. Low Plasma ApoE levels are associated with smaller hippocampal size in the Alzheimer's disease neuroimaging initiative cohort. Dement Geriatr Cogn Disord 2014; 39:154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer JH, Schuff N, Reed BR, et al. Hippocampal volume and retention in Alzheimer's disease. J Int Neuropsychol Soc 2004; 10:639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Rinne JO, Mosconi L, et al. Regional analysis of FDG and PIB-PET images in normal aging, mild cognitive impairment, and Alzheimer's disease. Eur J Nucl Med Mol Imaging 2008; 35:2169–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosconi L, Rinne JO, Tsui WH, et al. Increased fibrillar amyloid-{beta} burden in normal individuals with a family history of late-onset Alzheimer's. Proc Natl Acad Sci U S A 2010; 107:5949–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jack CR, Jr, Wiste HJ, Lesnick TG, et al. Brain beta-amyloid load approaches a plateau. Neurology 2013; 80:890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu LW, Chiu KC, Hui SL, et al. The reliability and validity of the Alzheimer's Disease Assessment Scale Cognitive Subscale (ADAS-Cog) among the elderly Chinese in Hong Kong. Ann Acad Med Singapore 2000; 29:474–485. [PubMed] [Google Scholar]
- 21.Huang CW, Chang WN, Lui CC, et al. Impacts of hyper-homocysteinemia and white matter hyper-intensity in Alzheimer's disease patients with normal creatinine: an MRI-based study with longitudinal follow-up. Curr Alzheimer Res 2010; 7:527–533. [DOI] [PubMed] [Google Scholar]
- 22.Huang CW, Chang WN, Huang SH, et al. Impact of homocysteine on cortical perfusion and cognitive decline in mild Alzheimer's dementia. Eur J Neurol 2013; 20:1191–1197. [DOI] [PubMed] [Google Scholar]
- 23.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011; 7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology 1993; 43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 25.Huang KL, Lin KJ, Hsiao IT, et al. Regional amyloid deposition in amnestic mild cognitive impairment and Alzheimer's disease evaluated by [18F]AV-45 positron emission tomography in Chinese population. PLoS One 2013; 8:e58974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen WG, Terry RD, Fuld PA, et al. Pathological verification of ischemic score in differentiation of dementias. Ann Neurol 1980; 7:486–488. [DOI] [PubMed] [Google Scholar]
- 27.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002; 15:273–289. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198. [DOI] [PubMed] [Google Scholar]
- 29.Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer's disease. J Neural Transm Suppl 1998; 53:127–140. [DOI] [PubMed] [Google Scholar]
- 30.Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA 2011; 305:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex 1992; 2:435–443. [DOI] [PubMed] [Google Scholar]
- 32.Sperling RA, Johnson KA, Doraiswamy PM, et al. Amyloid deposition detected with florbetapir F 18 ((18)F-AV-45) is related to lower episodic memory performance in clinically normal older individuals. Neurobiol Aging 2013; 34:822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheline YI, Raichle ME, Snyder AZ, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry 2010; 67:584–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR, Jr, Lowe VJ, Weigand SD, et al. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain 2009; 132:1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourgeat P, Chetelat G, Villemagne VL, et al. Beta-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology 2010; 74:121–127. [DOI] [PubMed] [Google Scholar]
- 36.Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci 2009; 21:489–510. [DOI] [PubMed] [Google Scholar]
- 37.Wolk DA, Dickerson BC. Fractionating verbal episodic memory in Alzheimer's disease. Neuroimage 2011; 54:1530–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golomb J, de Leon MJ, Kluger A, et al. Hippocampal atrophy in normal aging. An association with recent memory impairment. Arch Neurol 1993; 50:967–973. [DOI] [PubMed] [Google Scholar]


