Abstract
The aim of this study was to compare the efficacy and safety of S-1-based therapy versus non-S-1-based therapy in advanced gastric cancer (AGC) patients.
Eligible studies stratifying objective response rate (ORR), progression-free survival (PFS), overall survival (OS), and adverse events (AEs) in AGC patients were identified from Embase, Pubmed, Cochrane Library, and China National Knowledge Infrastructure databases. The STATA package (version 11.0) was used to pool the data from the eligible studies.
Fifteen studies with 2973 AGC cases, of which 1497 (50.4%) received S-1-based therapy and 1476 (49.6%) received non-S-1-based therapy, were identified in the meta-analysis. AGC patients who had received S-1-based therapy had a higher median OS, median PFS, and ORR than those who had received 5-fluorouracil (FU)-based therapy (OS: hazard ratio [HR] 0.89, 95% confidence interval [CI] 0.80–0.98, P = 0.015; PFS: HR 0.88, 95% CI 0.80–0.98, P = 0.016; ORR: OR 1.25, 95% CI 1.08–1.45, P = 0.003, respectively). S-1-based therapy had similar efficacy to capecitabine-based therapy in terms of median OS (HR 1.14, 95% CI 0.91–1.41, P = 0.253), median PFS (HR 1.01, 95% CI 0.82–1.25, P = 0.927), and ORR (OR 0.84, 95% CI 0.63–1.12, P = 0.226). Subgroup analysis for grade 3 to 4 toxicity showed higher incidence of neutropenia (relative risk [RR] = 0.827, P = 0.006), nausea (RR = 0.808, P = 0.040), and lower diarrhea (RR = 1.716, P = 0.012) in 5-FU-based arm, and higher diarrhea (RR = 0.386, P = 0.007) in capecitabine-based arm.
S-1-based chemotherapy is favorable to AGC patients with better clinical benefit than 5-FU-based chemotherapy and with equivalent antitumor compare with capecitabine-based therapy.
INTRODUCTION
Gastric cancer is one of the leading causes of death worldwide and prognosis is poor as symptoms often do not appear until the disease has reached an advanced stage.1,2 The incidence of gastric cancer is twice as high in men as women and the number of reported cases varies between countries. The number of deaths from gastric cancer has fallen over the past two decades, but it still ranks as the fourth most frequent cancer.1 Patients aged 65 years or older account for the most gastric cancer-related deaths.3 Although surgery and appropriate adjuvant chemotherapy are used in treatment, the prognosis is poor and average survival is <1 year.2
A previous early phase II clinical trial indicated that irinotecan plus cisplatin was beneficial to advanced gastric cancer (AGC) patients, reporting a response rate of 59% and median survival time of 322 days, but with a high incidence of grade 4 neutropenia (57%).4 Subsequent work showed that fluorouracil plus cisplatin contributed to a higher response rate and longer progression-free survival. This chemotherapy was used for more toxic events but did not extend survival compared with continuous infusion of fluorouracil alone. Therefore, more effective chemotherapeutic regimens are still required for the treatment of advanced gastric cancer.
In recent years, several phase III studies have been conducted for AGC, using a combination of 5-fluorouracil (5-FU) and cisplatin5,6; triple combinations using docetaxel or epirubicin have also been widely tested. A previous clinical trial indicated that irinotecan combined with folinic acid and 5-FU showed similar benefits to epirubicin plus cisplatin and capecitabine, but with a more tolerable toxicity.7 A significant improvement in survival was observed when using a combination of docetaxel plus 5-FU plus cisplatin, although the clinical benefit was limited and the regimen affected hematological toxicity.8
S-1 is an oral anticancer drug containing tegafur, gimeracil, and oteracil, which has been shown to improve anticancer activity and limit the gastrointestinal toxic effects of FU. The Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer (ACTS-GC) trial indicated that S-1-based therapy prolonged the survival of AGC patients further when compared with surgery alone.9 Previous phase II studies reported that S-1 monotherapy was beneficial for AGC patients with a response rate of 45% and a 2-year survival of 17%, with a 5% or lower incidence of grade 3 (or higher) toxic events.10 Combinations of S-1 and other cytotoxic drugs, such as docetaxel,11 irinotecan,12 and cisplatin,13 have been explored in several phase I/II studies. With higher objective response rates and lower frequencies of grade 3 or 4 toxic effects, these combinations are thought to be promising. S-1 plus cisplatin has been widely used for the treatment of AGC patients and as the standard chemotherapy regimen for AGC patients in Japan.14 This chemotherapy regimen is also being used in the European Union countries (EU) to treat AGC patients.15 A meta-analysis showed that S-1-based combination therapy could prolong overall survival (OS), progression-free survival (PFS) and improve objective response rate (ORR) with less toxicity for AGC patients, compared with S-1 monotherapy.16 A previous meta-analysis evaluated the efficacy and safety of S-1-based therapy versus 5-FU-based therapy in AGC and reported that S-1-based therapy extended OS with a lower incidence of grade 3 or grade 4 neutropenia, although there was no significant difference in ORR.17 This study did not analyze PFS and included only a limited number of eligible studies. Another meta-analysis study, of which 4 randomized controlled trials (RCTs) met our inclusion criteria, reported that S-1-based therapy had more clinical benefits than 5-FU-based therapy; however, the population in this study were all Chinese.18 In this study, a meta-analysis was performed on a set of eligible studies to investigate whether S-1-based therapy was more effective than non-S-1-based therapy for treating patients with AGC.
MATERIALS AND METHODS
Search Strategy
The Cochrane Library, PubMed, Embase, and China National Knowledge Infrastructure databases were searched to identify all relevant articles published before or on December 2, 2014 using the following key search terms: “S-1,” “Teysuno,” “TS-1,” “tegafur,” “gimeracil,” “oteracil,” “advanced stomach cancer,” “advanced stomach carcinoma,” “advanced gastric cancer,” “advanced gastric carcinoma,” “stomach neoplasm,” and “gastric neoplasm,” “treatment or chemotherapy”. The search was limited to human studies and without language restricted. We also searched for the references of all retrieved studies. In addition, Google Scholar search and all relevant abstracts from the American Society of Clinical Oncology (ASCO) conferences were conducted for supplementation. Finally, we manually selected relevant studies based on the summary analysis. The searches were performed independently by 2 investigators.
Eligibility Criteria
To be eligible for inclusion in the meta-analysis, all studies had to meet the following criteria: patients had pathologically proven AGC (unrespectable or metastatic) at baseline with no prior radiotherapy or previous adjuvant chemotherapy 1 month before starting the study; compared S-1-based therapy with other agent-based therapies as the first-line chemotherapy regimen; randomized controlled trials or retrospective studies; (4) reported data for calculating the efficacy or safety of these 2 chemotherapy regimens; and (5) presented or allowed the calculation of a hazard ratio (HR) and its 95% confidence intervals (CIs) for OS or PFS compared with 2 chemotherapy regimens. Data from case reports, review articles, and letters were not eligible for our study. When the same patient populations were published in several studies, only the most recent, largest, or complete study was included. Corresponding authors were contacted for more details if necessary. Two independent reviewers assessed all eligible articles using a standardized form.
Quality Assessment
Two independent reviewers used the Cochrane Handbook for Systematic Reviews of Interventions (version 5.0.2) to assess the quality of the RCTs.19 The Newcastle-Ottawa Quality Assessment Scale for cohort studies was used to evaluate the quality of the nonrandomized studies.20 Any disagreements were resolved by discussion between the investigators or consulting a third reviewer.
Data Extraction
The following data were collected from each eligible study: first author, ethnicity, publication year, number of patients evaluated, Eastern Cooperative Oncology Group (ECOG) performance status (PS), proportion of males and average age; (2) study design of the eligible studies, chemotherapy regimen, ORR, median PFS, median OS, and the HR of OS or PFS and its 95% CI; and (3) grade 3 or grade 4 adverse events (AEs). Two reviewers, working independently, used a standardized format to extract the data and this was checked for internal consistency. Consensus were resolved if any disagreement happened.
Statistical Analysis
The primary endpoints in our study were OS and PFS. The HR and its 95% CI were used to express the association between chemotherapy regimen and the primary endpoints. Either S-1-based therapy results in a shorter PFS or OS, with an HR of more than 1, or it leads to a longer PFS or OS with a HR of <1. The secondary endpoints in our study were ORR and AEs. ORR was defined as the sum of the complete and partial response rates according to the Response Evaluation Criteria in Solid Tumors.21 An odds ratio (OR) was used to represent the correlation between the chemotherapy regimen and the ORR of the S-1-based therapy arm over other agent or agent-based combination chemotherapy arms. Thus, there is no significant difference for the ORR between the 2 types of treatment when the OR is equal to 1; a favorable outcome in S-1-based therapy is an OR >1; the tendency of S-1-based patients to be less responsive to treatment is denoted by an OR <1. AEs of the eligible studies were evaluated using the National Cancer Institute's common toxicity criteria (version 2). A P value <0.05 was considered statistically significant. A fixed-effects model was conducted to pool HR or OR. We looked for heterogeneity using the traditional Q test and the I2 index based on standard methods.22 The source of heterogeneity was explored using the following techniques: sensitivity analysis, subgroup analysis, or the random-effects model.23 Begg test and Egger test were used to look for publication bias.24,25 All statistical analyses were performed using the META module of the STATA software program, version 11.0 (Stata Corporation, College Station, TX). A 2-tailed P value <0.05 was considered to be statistically significant.
RESULTS
Selection of Studies
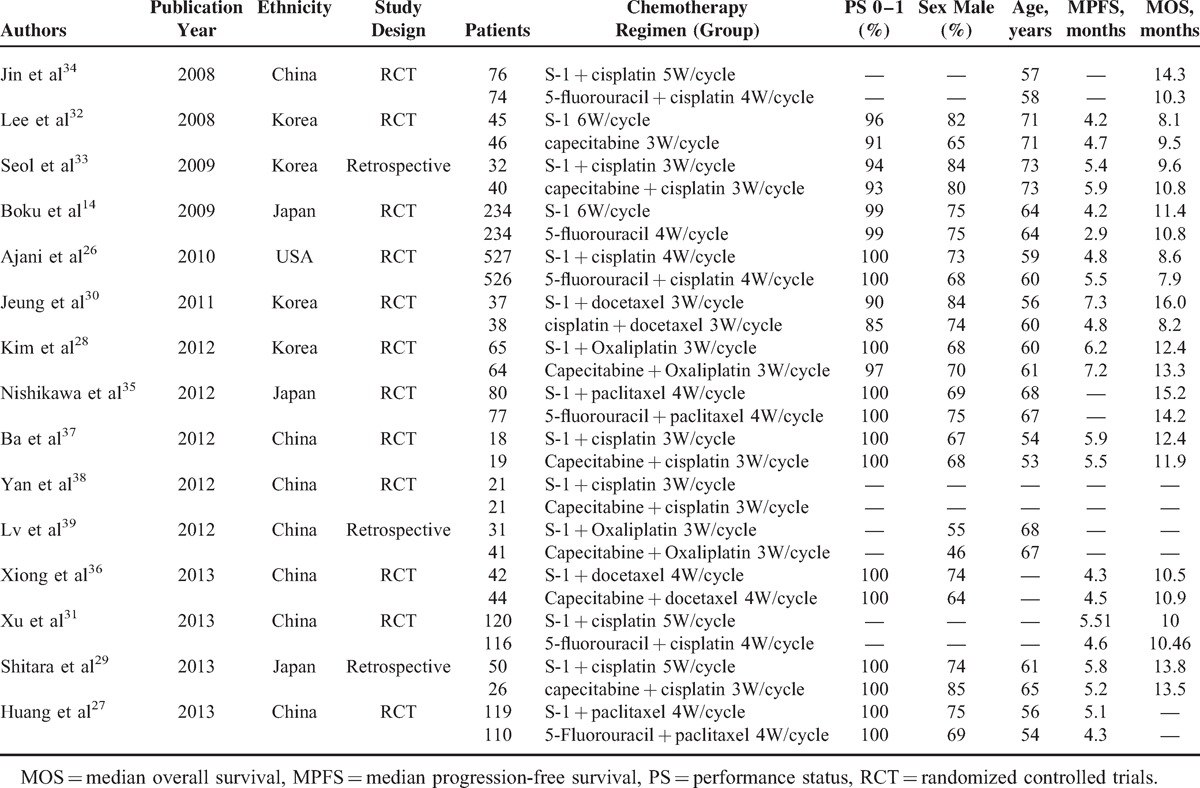
A total of 511 related publications were identified based on our initial screening without language restriction. After a careful review of the abstracts, 148 references were deemed eligible based on the inclusion criteria. After reviewing the complete articles, we excluded 64 studies based on the surgical treatment time-period, 35 studies that included concurrent chemo radiotherapy (CCRT) or radiotherapy, and 34 studies with inestimable data or unreachable authors. As a result, the meta-analysis included 15 studies14,26–39 involving 2973 AGC patients, with 1497 patients in the S-1-based therapy group (50.4%) and 1476 patients (49.6%) in the non-S-1 therapy group (Fig. 1). Four trail36–39 published in Chinese were retrieved from the references of He et al's study.40 The characteristics of the 10 included studies are displayed in Table 1.14,26–39
FIGURE 1.
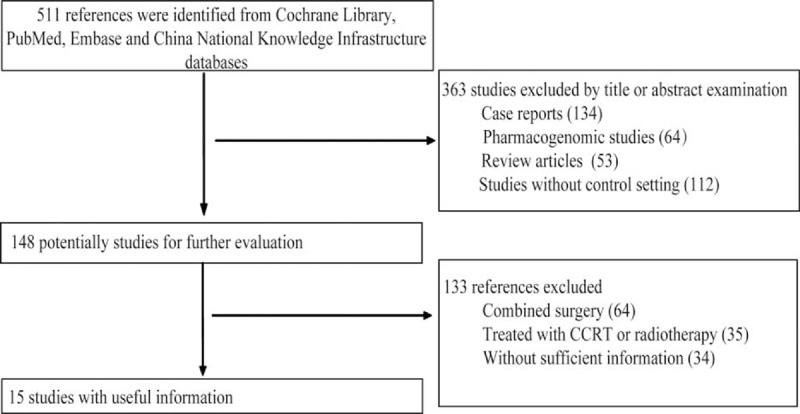
Flowchart of study selection.
TABLE 1.
Baseline Characteristics of Eligible Studies
Quality Assessment of the Studies
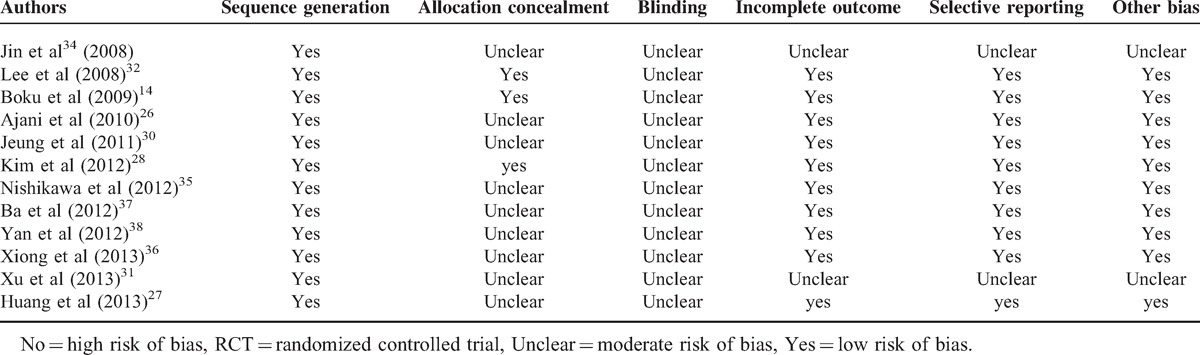
We used the Cochrane Handbook for Systematic Reviews of Interventions to assess the quality of the 12 included RCTs and all RCTs reported adequate generation of the allocation sequence. Three studies14,28,32 reported allocation concealment (concealed to the investigators). No trials reported a blinding process. Two studies31,34 we included are abstract, so the evaluation marks the incomplete outcome, selective reporting, and other bias as unclear. All the RCTs included in our study are level B. The Newcastle-Ottawa Quality Assessment Scale for cohort studies was used to assess the quality of three retrospective studies,29,33,39 resulting in high-quality scores with a total of 8 stars. The risk of bias for the 7 RCTs is listed in Table 2.14,26–28,30–32,34–38
TABLE 2.
Quality of RCTs Used in the Meta-analysis
Efficacy
Main Results of OS
Twelve eligible studies reported information for treatment and OS; the HR and its 95% CI for OS could be extracted from these studies. Univariate analysis was performed to calculate the HR and the corresponding 95% CI from the references for OS. We looked for some heterogeneity using the Q-test (χ2 = 13.56, P = 0.258, I2 = 18.9%) with the fixed-effects model. Pooled data from these 12 studies indicated that S-1-based therapy was favorable to AGC patients compared with non-S-1-based therapy (HR 0.92, 95% CI 0.85–0.99, P = 0.027) (Fig. 2). Subgroup analysis was performed based on chemotherapy regimen and study design. S-1-based therapy versus 5-FU-based therapy was conducted in 5 studies.14,26,31,34,35 The subgroup analysis indicated that AGC patients receiving S-1-based therapy experienced a longer OS compared with patients receiving 5-FU-based therapy (HR 0.89, 95% CI 0.80–0.98, P = 0.015) (Fig. 2A). Six studies were performed using capecitabine-based therapy28–30,33,36,37 and the subgroup analysis indicated that there was no significant difference in OS benefit (HR 1.01, 95% CI 0.89–1.15, P = 0.876) (Fig. 2A). Significant difference between S-1-based arm and non- S-1-based arm also was found in 10 RCTs14,26,28,30–32,34–37 (HR 0.91, 95% CI 0.84–0.98, P = 0.014) but not in 2 retrospective trials29,33 (HR 1.20, 95% CI 0.83–1.73, P = 0.336) (Fig. 2B). The results from a sensitivity analysis suggest that our findings are statistically robust. We did not observe any publication bias using either the funnel plot or Egger test (P = 0.304 and P = 0.587).
FIGURE 2.
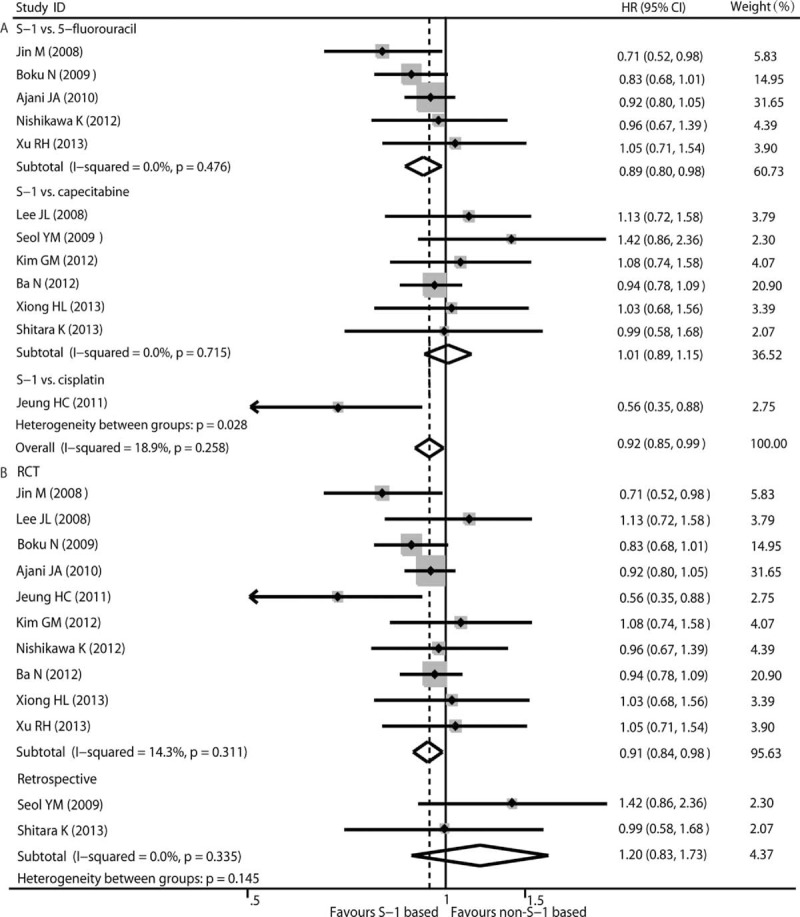
Pooled analyses and subgroup analysis (A, B) of OS associated with S-1-based therapy compared with non-S-1 therapy. HR with its 95% CI <1 indicate a longer OS for S-1 based chemotherapy. HR = hazard ratio, OS = overall survival, RCT = randomized controlled trials.
Main Results of PFS
Eleven eligible studies reported the HR with a 95% CI for PFS. The HR and corresponding 95% CI from references for PFS were pooled using univariate analysis. Some heterogeneity was observed using the Q test (χ2 = 13.32, P = 0.204, I2 = 25.2%) with the fixed-effects model. Pooled data of PFS indicated that AGC patients who received S-1-based therapy had a longer PFS than those who received non-S-1-based therapy (HR 0.90, 95% CI 0.83–0.97, P = 0.010) (Fig. 3). S-1-based therapy versus 5-FU-based therapy was conducted in 4 studies.14,26,27,31 Subgroup analysis with moderate heterogeneity (χ2 = 9.79, P = 0.020, I2 = 69.4%) indicated that S-1-based therapy showed a favorable outcome for AGC patients by prolonging PFS when compared with 5-FU-based therapy (HR 0.88, 95% CI 0.80–0.98, P = 0.016) (Fig. 3A). No heterogeneity was observed in the capecitabine-based therapy group (χ2 = 0.87, P = 0.973, I2 = 0.00%) and the pooled data indicated that S-1-based and capecitabine-based therapy showed a similar PFS benefit (HR 0.96, 95% CI 0.83–1.11, P = 0.567) (Fig. 3A). Significant difference between S-1-based arm and non-S-1-based arm also was found in 9 RCTs14,26–28,30–32,36,37 (HR 0.89, 95% CI 0.82–0.97, P = 0.007) but not in 2 retrospective trials29,33 (HR 1.03, 95% CI 0.75–1.43, P = 0.835) (Fig. 3B). The results from a sensitivity analysis suggest that our findings are statistically robust. No publication bias was detected using either the funnel plot or Egger test (P = 0.436 and P = 0.719).
FIGURE 3.
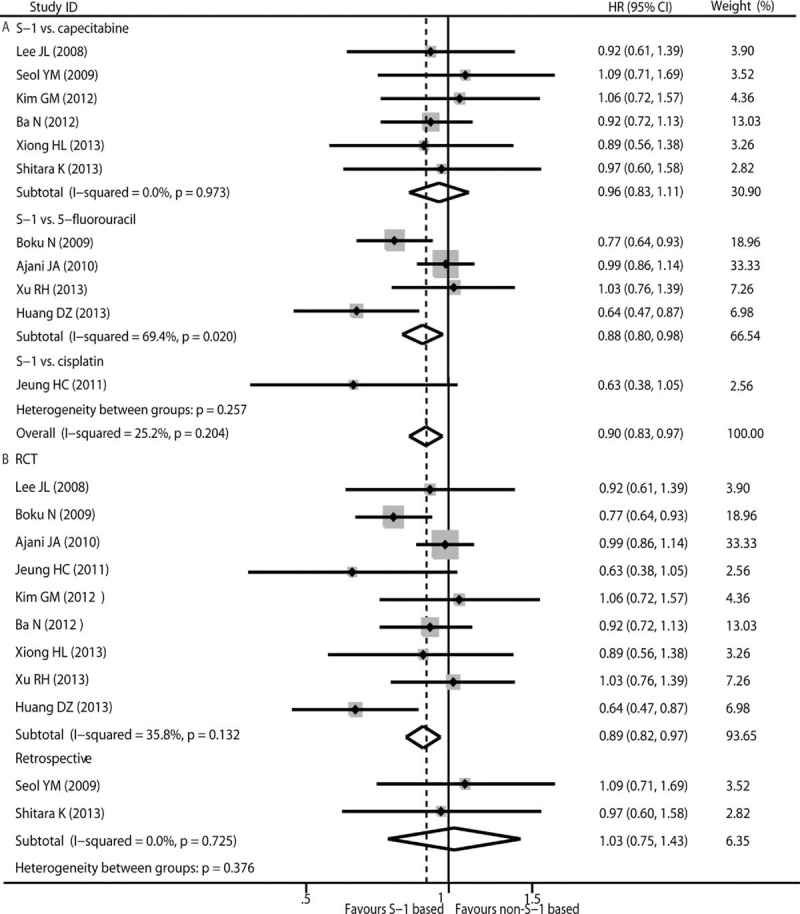
Pooled analyses and subgroup analysis (A, B) of PFS associated with S-1-based therapy compared with non-S-1 therapy. HR with its 95% CI <1 indicate a longer PFS for S-1 based chemotherapy. HR = hazard ratio, PFS = progression-free survival, RCT = randomized controlled trials.
Overall Response Rate
Tumor objective responses were extracted from all eligible studies, which included 2444 patients. We looked for moderate heterogeneity across studies using the fixed-effects model (χ2 = 40.12, P = 0.00; I2 = 65.1%), and the subgroup analysis showed that 5-FU-based chemotherapy (χ2 = 31.36, P = 0.00; I2 = 84.1%) was the main sources of heterogeneity. The ORR of AGC patients with S-1-based therapy was 33.20% (413/1244), whereas that of AGC patients with non-S-1-based regimens was 28.17% (338/1200), which indicated that S-1-based therapy could improve ORR for AGC patients compared with non-S-1-based therapy (OR = 1.17; 95% CI 1.04–1.31; P = 0.011) (Fig. 4). In the subgroup analysis of the chemotherapy regimen, the ORR for patients who received S-1-based therapy was higher than that of patients who received 5-FU-based therapy (P = 0.003) (Fig. 4A). There was no heterogeneity (χ2 = 2.02, P = 0.958, I2 = 0.0%) in the capecitabine-based therapy group and the result indicated that capecitabine-based therapy was not superior to S-1-based therapy in overall response rate (P = 0.433) (Fig. 4A). Significant difference between S-1-based arm and non-S-1-based arm also was found in 12 RCTs14,26–28,30–32,34–38 (HR 1.20, 95% CI 1.06–1.36, P = 0.004) but not in 3 retrospective trials29,33,39 (HR 0.85, 95% CI 0.59–1.24, P = 0.407) (Fig. 4B). The results from a sensitivity analysis suggest that our findings are statistically robust. No publication bias was detected using either the funnel plot or Egger test (P = 0.553 and P = 0.507).
FIGURE 4.
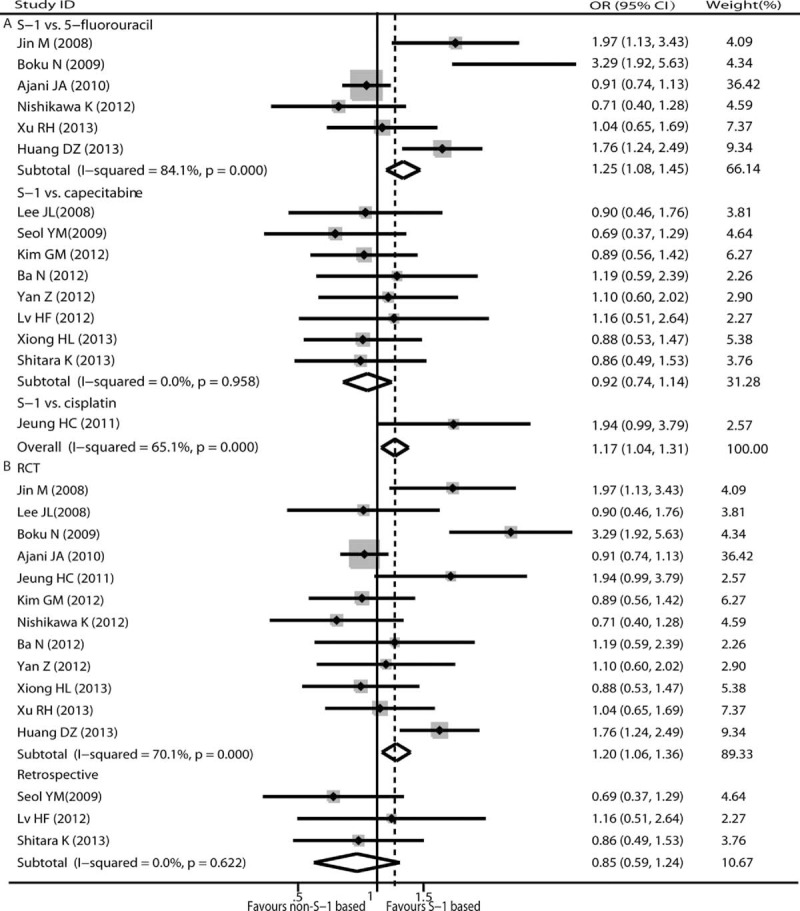
Pooled analyses and subgroup analysis (A, B) of ORR associated with S-1-based therapy compared with non-S-1 therapy. OR with its 95% CI >1 indicates a higher ORR for S-1 based chemotherapy. OR = odds ratio, ORR = objective response rate, RCT = randomized controlled trials.
Analysis for non-S1-based Therapy (5-FU vs Capecitabine)
The statistical method published by Altman et al41 was used to conducted meta-analysis among non-S1-based therapy, which indicated that similar efficacy of OS (HR 0.88, 95% CI 0.75–1.03) and PFS (HR 1.09, 95% CI 0.91–1.30) was found between 5-FU-based arm and capecitabine-based arm other than capecitabine-based arm had higher ORR (relative risk [RR] 1.36, 95% CI 1.05–1.76).
Toxicity
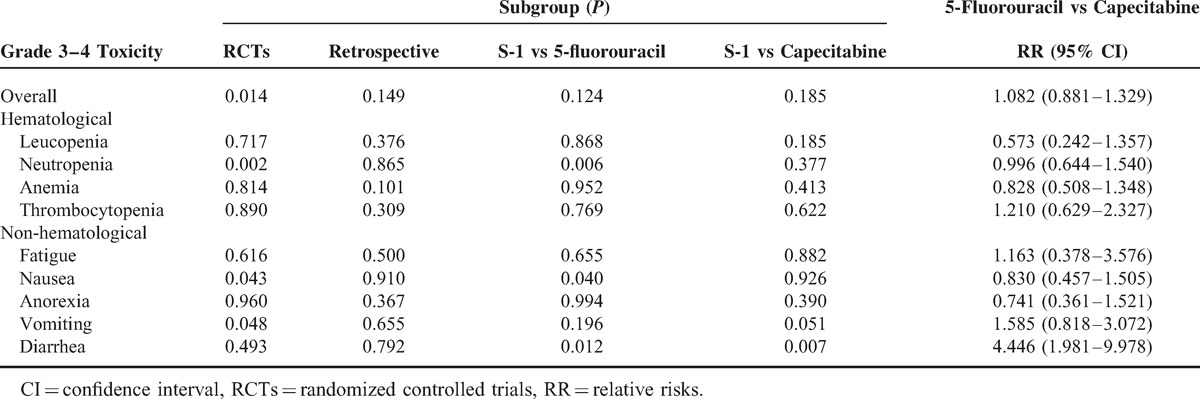
The toxicity profile analyses for eligible trials are shown in Table 3. The most common grade 3 to 4 hematologic toxicities were neutropenia and anemia in both arms, and the most frequent grade 3 to 4 nonhematological toxicities were nausea for each arm. Pooled data revealed no significant difference in safety profiles for both grade 3 to 4 hematologic and grade 3 to 4 nonhematological events, other than lower incidence of neutropenia and vomiting were observed in S-1-based arm. Subgroup analysis for grade 3 to 4 toxicity showed higher incidence of neutropenia (RR = 0.827, P = 0.006) and nausea (RR = 0.808, P = 0.040), lower diarrhea (RR = 1.716, P = 0.012) in 5-FU-based arm, and higher diarrhea (RR = 0.386, P = 0.007) in capecitabine-based arm. Analysis among non-S1-based arm, which was performed via the method of Altman et al literature,41 showed that 5-FU-based arm had lower incidence of diarrhea than that in capecitabine-based arm (RR 4.45, 95% CI 1.98–9.98) (Table 4).
TABLE 3.
Outcome of Grade 3–4 Toxicity Meta-analysis
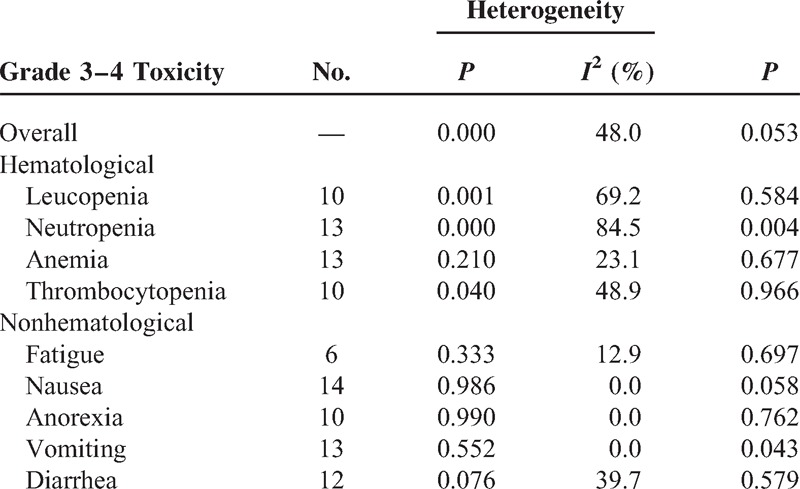
TABLE 4.
Subgroup Analyses for Grade 3–4 Toxicity
DISCUSSION
Several early phase II and III studies have reported that S-1 has a multifactorial synergistic effect on anti-tumor activity in AGC patients in a single agent setting.10,42 In addition, several trials exploring combinations of S-1 and other agents for the treatment of AGC have been conducted widely in Asian countries including Japan, China, and Korea. However, as yet there is no consensus in the medical community regarding the superiority of S-1-based therapy over other agent or agent-based therapies for AGC patients. The meta-analysis of published data presented in this study aimed to address this by determining whether S-1-based therapy could improve objective response rate, control symptoms, prolong survival, and maintain or improve quality of life compared with non-S-1-based therapy in patients with AGC.
The results from our study indicate that S-1-based chemotherapy showed greater clinical benefit in terms of OS, PFS, and ORR; achieved tolerability 3 to 4 toxicity for AGC patients when compared with 5-FU-based therapy, and has similar clinical benefit with capecitabine-based therapy. In the subgroup analysis of the chemotherapy regimen for OS, S-1-based therapy prolonged the OS in AGC patients compared with 5-FU-based therapy. This finding concurred with a previous meta-analysis conducted by Huang et al.17 We note that Fuse et al's study43 is the updated analysis of Boku et al's study14 with the same patients population and both of these studies were included in the Huang et al's study17; with careful consideration, the study conducted by Fuse et al43 was excluded from our study because of insufficient information. Furthermore, the results in our study were generated from a larger sample size and a greater number of studies; therefore it could be considered more statistically robust than the aforementioned study.17
When S-1-based therapy was compared with 5-FU-based therapy with regard to PFS, the former was found to be more effective (P = 0.016) for AGC patients. This particular finding was not reported in the previous meta-analysis by Huang et al.17 With regard to the ORR, the Huang et al's study17 reported that S-1-based therapy was not superior to 5-FU-based therapy (OR = 1.25, 95% CI 0.31–5.09, P = 0.734). However, our results from the subgroup analysis indicated the contrary. The reasons for this might be that only 2 studies provided relevant information of overall response rate in the Huang et al's study,17 whereas we found 6 such studies. Furthermore, the data extracted from the trial by Boku et al's study14 regarding the ORR in the 5-FU-based therapy group that is referenced in the Huang17 study should be 15/175, not 68/181. Another meta-analysis,44 which included the same 6 articles with our current analysis, used randomized-effects model to pool OR for ORR among S-1-based and 5-FU-based because high heterogeneity was observed, with the results that no significant difference was found in terms of ORR between these 2 arms. It seems that the total studies size of six might not warrant using randomized-effects model; therefore, fixed-effects model was conducted and the result indicated that S-1-based arm had higher ORR than the 5-FU-based arm. This result was consistent with Yang et al's.45 The purpose of our study is consistent with Yang et al’ study45 with similar conclusions, but more related RCTs were included in our current trail.
There was insufficient information on safety profiles in 1 eligible study,30 even after contacting the author. The pooled data on toxic effects indicated no significant difference in most of grade 3 to 4 toxic events between S-1-based therapy and non-S-1-based therapy, and all the toxicities were manageable, tolerable, and predictable. Although high heterogeneity was found in subgroup analysis for grade 3 to 4 neutropenia, the main sources of heterogeneity came from 5-FU-based chemotherapy group including 6 literatures; therefore, we still used fixed-effects model to perform analysis.
Two previous meta-analysis articles40,46 showed that S-1-based treatment had similar antitumor efficacy with capecitabine-based treatment for AGC patients; 4 trials included in them were not included in our current analysis because those 4 trails clearly stated that S-1 provided to the AGC patients was made in china, which might lead to clinical heterogeneity. Conclusions from other meta-analysis studies have indicated that S-1-based and capecitabine-based chemotherapy treatments are similarly effective and well tolerated for gastrointestinal cancers47; in this case, our work is limited to advanced gastric cancer; therefore, 2 studies related to colorectal cancer were excluded from our work. We noted that the conclusion we drew from the capecitabine-based therapy subgroup analysis is similar to that of the 3 meta-analysis studies mentioned above.
There are some clear weaknesses in our study. First, we used the method posed by Altman et al41 to assess the efficacy and tolerability of non-S-1-based chemotherapy among AGC patients, and our results showed that higher ORR and grade 3 to 4 diarrhea were observed in capecitabine-based arm compared with 5-fluorouracil-based arm. This conclusion needs to be confirmed because these calculations are used for comparing 2 estimated RRs from subgroup analysis. Second, the eligible studies included 12 RCTs with level B in the quality assessment and three studies29,33,39 with higher score, and the results could have been affected by the quality of the individual studies. Finally, most of the studies in our analysis comprised populations from East Asia who have a similar genetic background; only 1 study was conducted in the West (USA).26 Therefore, we did not perform a subgroup analysis based on ethnicity, and these conclusions should be confirmed via high-quality RCTs and Western studies.
Overall, our study shows that S-1-based chemotherapy is favorable to AGC patients with better clinical benefit in term of OS, PFS, and ORR when compared with 5-FU-based chemotherapy and have similar antitumor efficacy compared with capecitabine-based chemotherapy. Financially, S-1 is cheaper than continuous infusion of fluorouracil and it benefits from the convenience of oral administration. We would therefore recommend S-1-based therapy as a chemotherapeutic regimen for AGC patients in future, once our findings have been confirmed through larger studies and further clinical trials.
Footnotes
Abbreviations: AGC = advanced gastric cancer, ORR = objective response rate, PFS = progression-free survival, OS = overall survival, AEs = adverse events, 5-FU = 5-fluorouracil, ACTS-GC = Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer, EU = European Union countries, ASCO = American Society of Clinical Oncology, HR = hazard ratio, CI = confidence intervals, PS = performance status, ECOG = Eastern Cooperative Oncology Group, OR = odds ratio, RCTs = randomized controlled trials, RR = relative risks.
F-LW, D-CL, Y-PY contributed equally to the writing of this article and they should be regarded as joint first authors.
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Brenner H, Rothenbacher D, Arndt V. Epidemiology of stomach cancer. Methods Mol Biol (Clifton, NJ) 2009; 472:467–477. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. Cancer J Clin 2012; 62:10–29. [DOI] [PubMed] [Google Scholar]
- 3.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2010, National Cancer Institute; Bethesda, MD, http://seercancergov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, April 2013. 2013. [Google Scholar]
- 4.Boku N, Ohtsu A, Shimada Y, et al. Phase II study of a combination of irinotecan and cisplatin against metastatic gastric cancer. J Clin Oncol 1999; 17:319–323. [DOI] [PubMed] [Google Scholar]
- 5.Ajani JA, Moiseyenko VM, Tjulandin S, et al. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol 2007; 25:3205–3209. [DOI] [PubMed] [Google Scholar]
- 6.Dank M, Zaluski J, Barone C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol 2008; 19:1450–1457. [DOI] [PubMed] [Google Scholar]
- 7.Delaunoit T. Latest developments and emerging treatment options in the management of stomach cancer. Cancer Manag Res 2011; 3:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006; 24:4991–4997. [DOI] [PubMed] [Google Scholar]
- 9.Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007; 357:1810–1820. [DOI] [PubMed] [Google Scholar]
- 10.Koizumi W, Kurihara M, Nakano S, et al. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology 2000; 58:191–197. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida K, Ninomiya M, Takakura N, et al. Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res 2006; 12:3402–3407. [DOI] [PubMed] [Google Scholar]
- 12.Uedo N, Narahara H, Ishihara R, et al. Phase II study of a combination of irinotecan and S-1 in patients with advanced gastric cancer (OGSG0002). Oncology 2007; 73:65–71. [DOI] [PubMed] [Google Scholar]
- 13.Kimura M, Usami E, Ito D, et al. Tolerable evaluation for chemotherapy with S-1 plus cisplatin in elderly patients with advanced and recurrent gastric cancer. Gan To Kagaku Ryoho 2012; 39:1209–1214. [PubMed] [Google Scholar]
- 14.Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 2009; 10:1063–1069. [DOI] [PubMed] [Google Scholar]
- 15.Nordic Group BV. Teysuno 15 mg/4.35 mg/11.8 mg hard capsules: summary of product characteristics. http://wwwemaeuropaeu/docs/en_GB/document_library/EPAR-Product_Information/human/001242/WC500104415pdf. 2011: Accessed at 15 March 2014. [Google Scholar]
- 16.Liu GF, Tang D, Li P, et al. S-1-based combination therapy vs S-1 monotherapy in advanced gastric cancer: A meta-analysis. World J Gastroenterol 2014; 20:310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J, Cao Y, Wu L, et al. S-1-based therapy versus 5-FU-based therapy in advanced gastric cancer: a meta-analysis. Med Oncol (Northwood, London, England) 2011; 28:1004–1011. [DOI] [PubMed] [Google Scholar]
- 18.Chen XD, Tang LC, Tang XL, et al. A systematic review of S-1-based therapy versus 5-FU-based therapy in Chinese patients with advanced gastric cancer. Transl Gastrointest Cancer 2013; 2:AB61. [Google Scholar]
- 19.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. [updated September 2008]. The Cochrane Collaboration. 2009. [Google Scholar]
- 20.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analysis.. http://wwwohrica/programs/clinical_epidemiology/oxfordasp Accessed 1 Nov 2013. 2003. [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000; 92:205–216. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 26.Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 2010; 28:1547–1553. [DOI] [PubMed] [Google Scholar]
- 27.Huang DZ, Ba Y, Xiong J, et al. A multicentre randomised trial comparing weekly paclitaxel + S-1 with weekly paclitaxel + 5-fluorouracil for patients with advanced gastric cancer. Eu J Cancer (Oxford, England: 1990) 2013; 49:2995–3002. [DOI] [PubMed] [Google Scholar]
- 28.Kim GM, F HC, Rha SY, et al. A randomized phase II trial of S-1-oxaliplatin versus capecitabine-oxaliplatin in advanced gastric cancer. Eur J Cancer (Oxford, England: 1990) 2012; 48:518–526. [DOI] [PubMed] [Google Scholar]
- 29.Shitara K, Sawaki A, Matsuo K, et al. A retrospective comparison of S-1 plus cisplatin and capecitabine plus cisplatin for patients with advanced or recurrent gastric cancer. Int J Clin Oncol 2013; 18:539–546. [DOI] [PubMed] [Google Scholar]
- 30.Jeung HC, Rha SY, Im CK, et al. A Randomized phase 2 study of docetaxel and s-1 versus docetaxel and cisplatin in advanced gastric cancer with an evaluation of SPARC expression for personalized therapy. Cancer 2011; 117:2050–2057. [DOI] [PubMed] [Google Scholar]
- 31.Xu RH, Sun GP, Lu HS, et al. A phase III study of S-1 plus cisplatin versus fluorouracil plus cisplatin in patients with advanced gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol 2013; 15 (Suppl):11. [Google Scholar]
- 32.Lee JL, Kang YK, Kang HJ, et al. A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Brit J Cancer 2008; 99:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seol YM, Song MK, Choi YJ, et al. Oral fluoropyrimidines (capecitabine or S-1) and cisplatin as first line treatment in elderly patients with advanced gastric cancer: a retrospective study. Jpn J Clin Oncology 2009; 39:43–48. [DOI] [PubMed] [Google Scholar]
- 34.Jin M, Lu H, Li J, et al. Randomized 3-armed phase III study of S-1 monotherapy versus S-1/CDDP (SP) versus 5-FU/CDDP (FP) in patients (pts) with advanced gastric cancer (AGC): SC-101 study [abstract]. J Clin Oncol 2008; 26:4533. [Google Scholar]
- 35.Nishikawa K, Morita S, Matsui T, et al. A randomized phase-II trial comparing sequential and concurrent paclitaxel with oral or parenteral fluorinated pyrimidines for advanced or metastatic gastric cancer. Gastric Cancer 2012; 15:363–369. [DOI] [PubMed] [Google Scholar]
- 36.Xiong HL, Liu XQ, Sun AH, et al. Clinical comparison of Docetaxel combined with S-1 or Capicitabine in treating advanced gastric carcinoma. Med Oncol (Northwood, London, England) 2013; 21:581–584. [Google Scholar]
- 37.Ba Z, Wu M, Wang LJ, et al. Curative effect analysis of S-1 combined with cisplatin or capecitabine combined with cisplatin in the treatment of advanced gastric cancer. Chinese General Pract 2012; 15:672–676. [Google Scholar]
- 38.Yan Z, Zhao TX, Xing Y, et al. Curative effect analysis of S-1 combined with cisplatin in the treatment of advanced gastric cancer. Progress Mod Biomed 2012; 12:5324–5326. [Google Scholar]
- 39.Lv HF, Zhang YX, Han LL, et al. Clinical observation of Oxaliplatin combined with Gimeracil or Capecitabine in the treatment of advanced gastric cancer in elderly patients. China Med Herald 2012; 9:61–63. [Google Scholar]
- 40.He MM, Wu WJ, Wang F, et al. S-1-based chemotherapy versus capecitabine-based chemotherapy as first-line treatment for advanced gastric carcinoma: a meta-analysis. PLoS One 2013; 8:e82798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altman DG, Bland JM. Statistics Notes Interaction revisited: the difference between two estimates. BMJ (Clinical Research ed) 2003; 326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chollet P, Schoffski P, Weigang-Kohler K, et al. Phase II trial with S-1 in chemotherapy-naive patients with gastric cancer. A trial performed by the EORTC Early Clinical Studies Group (ECSG). Eur J Cancer (Oxford, England: 1990) 2003; 39:1264–1270. [DOI] [PubMed] [Google Scholar]
- 43.Fuse N, Fukuda H, Yamada Y, et al. Updated results of randomized phase III study of 5-fluorouracil (5-FU) alone versus combination of irinotecan, cisplatin (CP) versus S-1 alone in advanced gastric cancer (JCOG 9912) [abstract]. J Clin Oncol 2009; 27:15S. [Google Scholar]
- 44.Li DH, Pan ZK, Ye F, et al. S-1-based versus 5-FU-based chemotherapy as first-line treatment in advanced gastric cancer: a meta-analysis of randomized controlled trials. Tumour Biol 2014; 35:8201–8208. [DOI] [PubMed] [Google Scholar]
- 45.Yang J, Zhou Y, Min K, et al. S-1-based vs non-S-1-based chemotherapy in advanced gastric cancer: a meta-analysis. World J Gastroenterol 2014; 20:11886–11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye JX, Liu AQ, Ge LY, et al. Effectiveness and safety profile of S-1-based chemotherapy compared with capecitabine-based chemotherapy for advanced gastric and colorectal cancer: A meta-analysis. Exp Ther Med 2014; 7:1271–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Cao C, Zhang Q, et al. Comparison of the efficacy and safety of S-1-based and capecitabine-based regimens in gastrointestinal cancer: a meta-analysis. PLoS One 2014; 9:e84230. [DOI] [PMC free article] [PubMed] [Google Scholar]


