Abstract
Complicated infectious spondylitis is an infrequent infection with severe spinal destruction, and is indicated for combined anterior and posterior surgeries. Staged debridement and subsequent reconstruction is advocated in the literature. The purpose of this study is to evaluate the feasibility and clinical outcome of patients who underwent single-stage combined anterior debridement and fibular allograft implantation followed by supplemental posterior fixation for complicated infectious spondylitis.
We retrospectively reviewed the medical records of 20 patients who underwent single-stage combined anterior and posterior surgeries for complicated infectious spondylitis from January 2005 to December 2010. Complicated infectious spondylitis was defined as at least 1 vertebral osteomyelitis with pathological fracture or severe bony destruction and adjacent discitis, based on imaging studies. The severity of the neurological status was evaluated using the Frankel scale. The clinical outcomes were assessed by careful physical examination and regular serological tests to determine the visual analog scale (VAS) score and Macnab criteria. Correction of the sagittal Cobb angle on radiography was also compared before and after surgery. The Wilcoxon signed-rank test was used to analyze patient surgical prognosis and radiological findings.
All patients with complicated infectious spondylitis were successfully treated by single-stage combined anterior and posterior surgeries. No patients experienced neurologic deterioration. The average VAS score was 7.8 before surgery and significantly decreased to 2.1 at discharge. Three patients had excellent outcomes and 17 had good outcomes, based on Macnab criteria. The average length of the allograft for reconstruction was 64.0 mm. Kyphotic deformity improved in all patients, with an average correction angle of 13.4°. There was no implant breakage or allograft dislodgement during at least 36 months of follow-up.
Single-stage anterior debridement and fibular allograft implantation followed by posterior pedicle screw instrumentation provide immediate stability, satisfactory alignment, and successful infection control. Fibular allograft implantation seems to be a good alternative for anterior reconstruction; it can proceed to bony incorporation and avoids donor site morbidity.
INTRODUCTION
Spinal infections can be traced back as early as ancient Egypt, as spinal tuberculosis was identified in mummies. Since then, spinal infections have continued to be a challenge for clinical physicians because of their varied presentation and complicated course.1–4 Tuberculous spondylitis remains a major problem in underdeveloped and developing countries and is resurgent in the Western world; it can induce severe disability with paraplegia and kyphotic deformity. Pyogenic spondylitis is the most common spinal infection and can spontaneously occur in immunocompromised patients as a result of hematogenous spread from other inflammatory foci or following diagnostic and therapeutic procedures.5–8 The typical clinical symptoms and signs of infectious spondylitis, either pyogenic or tuberculous spondylitis, include severe back pain with or without sciatica. Advanced or complicated spinal infection can lead to structural instability, spinal deformity, sepsis, neurologic deficit, and even death.
In cases of complicated osteomyelitis in long bones, the treatment principles are extensive debridement and associated temporary stabilization. After the infection is under control, an optimal-size autograft is implanted to fit the bony defect of the lesion area and definite fixation is then performed. If the bony defect is >6 cm in length, a vascular bone graft should be considered as the treatment of choice.9–11 The anatomical specialty and axial-loading characteristics make the task of spinal reconstruction more difficult and complex. There is no guideline regarding the use of bone graft in the management of bony defect in complicated infectious spondylitis. The purpose of this study was to evaluate the feasibility and clinical outcomes of patients with complicated infectious spondylitis, who underwent single-stage combined anterior debridement accompanied with strut fibular allografting and supplemental posterior fixation.
METHODS
Study Population
This study was approved by the Institutional Review Board of E-DA Hospital, I-Shou University, Kaohsiung, Taiwan, a 1200-bed tertiary referral center (IRB number: EMRP-101-027). A total of 1936 patients undergoing spine surgery between January 2005 and December 2010 at our university hospital were screened in this study. Twenty-two of them had complicated infectious spondylitis and underwent single-stage combined anterior and posterior surgeries. The patients’ medical records, including outpatient and emergency room notes, admission notes, inpatient progress and nursing notes, discharge summaries, procedure notes, surgical reports, radiology reports, pathology reports, and microbiology laboratory results were reviewed. Infectious spondylitis was diagnosed based on clinical examinations, including positive physical or neurological presentations, elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values, and radiographic and magnetic resonance imaging (MRI) findings. Complicated infectious spondylitis was defined as at least 1-level vertebral osteomyelitis with pathological fracture or severe bony destruction and adjacent discitis, based on imaging studies. Two patients were excluded from this study due to loss of follow-up after surgery. The 20 patients with complicated infectious spondylitis enrolled in this study met the surgical indications of failed conservative treatment, neurological compromise, and spinal instability or kyphotic deformity. There were 7 women and 13 men, with an average age of 59.6 years (range, 39–77 years). These patients wore a rigid orthosis for protection at least 3 months after surgery. Radiographic assessment was performed before and after surgery, and at the 3, 6, and 12-month visit after discharge and every year thereafter. At 12 months after surgery, a computed tomography scan was taken to determine union status or bony incorporation between the allograft and the host bone. Systemic antibiotics or anti-tuberculous drugs were administered based on sensitivity studies for identified pathogens. A 6-week full course of intravenous antibiotics was prescribed for pyogenic spondylitis, and a 12-month full course of antimicrobial chemotherapy for tuberculous spondylitis. All 20 enrolled patients were followed up for at least 36 months after undergoing single-stage combined anterior and posterior surgeries.
Surgical Technique
All patients underwent single-stage combined anterior and posterior surgeries carried out by our spinal team. Fibular allogenous bone grafts were provided by the bone bank at our university hospital. The sequence of anterior or posterior surgery depended on the spinal stability and neurological status of the individual.
Anterior Surgery
All patients were operated under general anesthesia with endotracheal intubation; the vital signs of the patients, including heart rhythm, blood pressure, and pulse oxygenation levels, were continually monitored by anesthesiologists during the operation. Patients were placed in the right lateral decubitus position to enable an approach to the thoracic, thoracolumbar, or lumbar spine. The infected lesion was radically debrided and all destroyed tissues were removed. The spinal cord and neural elements were also completely decompressed. Smear, bacterial, and tuberculous cultures were done. The local area was thoroughly irrigated with diluted betadine and normal saline solution. During the procedure, a surgical assistant pushed the back anteriorly to correct the kyphotic spine, and the length of the bone graft was determined. A tubular fibular allograft of adequate length was then procured and prepared. Multiple drilling using Kirschner wires was performed on both ends of the allograft to facilitate bony incorporation. The kyphotic spine was again reduced by the surgical assistant and the bone graft was implanted into the space between the healthy vertebral bodies for anterior support and axial loading. The wound was closed with drain insertion.
Posterior Surgery
Under general anesthesia with endotracheal intubation, all patients were placed on the 4-poster bed frame in the prone position. The posterior elements of the spine were kept as intact as possible. The transpedicle screw instrumentation was performed at least 2 segments above the lesion and 2 below, for immediate stability. The devices used for instrumentation included Moss-Miami (DePuy, Raynham, MA), Tri-Fix (A-Spine, Taipei, Taiwan), and Clickx (Synthes, Solothurn, Switzerland). The levels of posterior supplemental transpedicle screw instrumentation were based on the clinical situation, including bone quality, spinal stability, infection severity, and imaging findings detected by either radiograph or MRI. After thorough irrigation with diluted betadine and normal saline solution, the wound was closed with drain insertion.
Outcome Assessment
The severity of the neurological status was evaluated using the Frankel scale before surgery and at discharge. The correction of the sagittal Cobb angle before and after surgery was compared using radiographic examination images. The Cobb angle, defined as the angle between the superior endplate of the vertebrae above the implanted allograft and the inferior endplate of the vertebrae below, was measured on plain lateral radiographs. The clinical outcomes were assessed by asking patients to qualify their pain on a visual analog scale (VAS, using a scale of 0–10; 0 means no pain and 10 means the most pain possible) and by careful physical examination and regular serological tests during admission and before discharge to determine the Macnab criteria. The Frankel scale, sagittal Cobb angle, and VAS before and after surgery were compared and analyzed using the Wilcoxon signed-rank test. Nonparametric statistics were used because some variables did not have normally distributed data. SPSS 13.0 software (SPSS Inc, Chicago, IL) was used for data analysis. A value of P < 0.05 was considered statistically significant.
RESULTS
Seven patients underwent anterior debridement with 1-level corpectomy and adjacent discectomies, 10 patients with 2 levels, 2 patients with 3 levels, and 1 patient with 4 levels. Eighteen patients underwent 2 levels above and 2 levels below instrumentation of the anterior corpectomy and allograft reconstruction, and 2 patients underwent 3 levels above and 3 levels below (Figures 1–6). Neurologic deficits were much improved, from the median of Frankel D before surgery to Frankel E before discharge (P = 0.001). Only 4 patients still had abnormal neurologic function before discharge, including Frankel D in 3 patients and Frankel C in 1. No patients experienced neurologic deterioration after operation. The sagittal Cobb angle was significantly changed, from the mean angle of −8.7° (range, −40° to 12°) preoperatively to 4.7° (range, −19° to 28°) postoperatively (P < 0.001). (Minus means the sagittal kyphotic angle as opposed to the sagittal lordotic angle plus.) The most prominent clinical sign of infectious spondylitis was severe back pain, which significantly decreased from the average of VAS 7.8 (range, 7–9) before surgery to VAS 2.1 (range, 1–3) before discharge (P < 0.001). Three patients had excellent outcomes and 17 had good outcomes based on Macnab criteria (Table 1).
FIGURE 1.
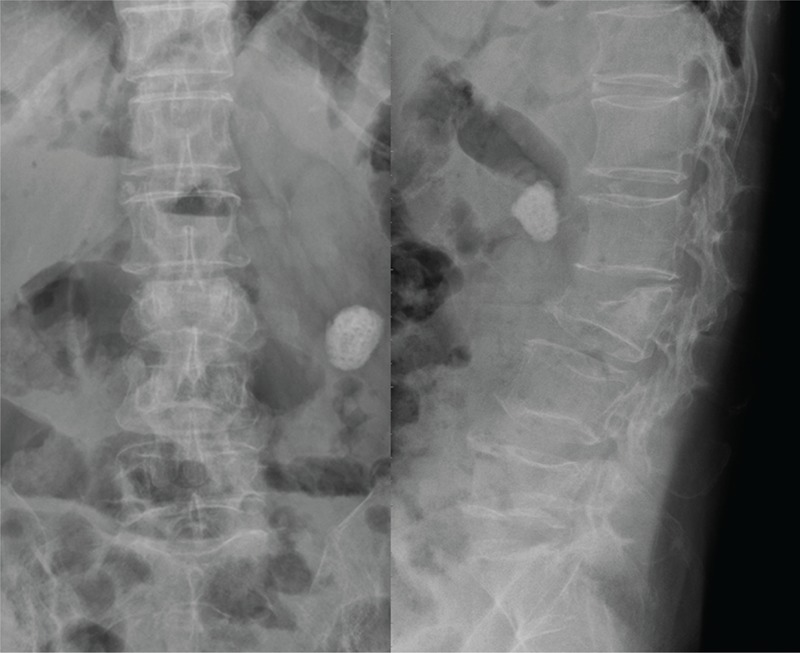
A 60-year-old women suffered from L3 intractable back pain and intermittent high fever. The radiograph showed L3 pathological fracture with superior endplate erosion.
FIGURE 6.
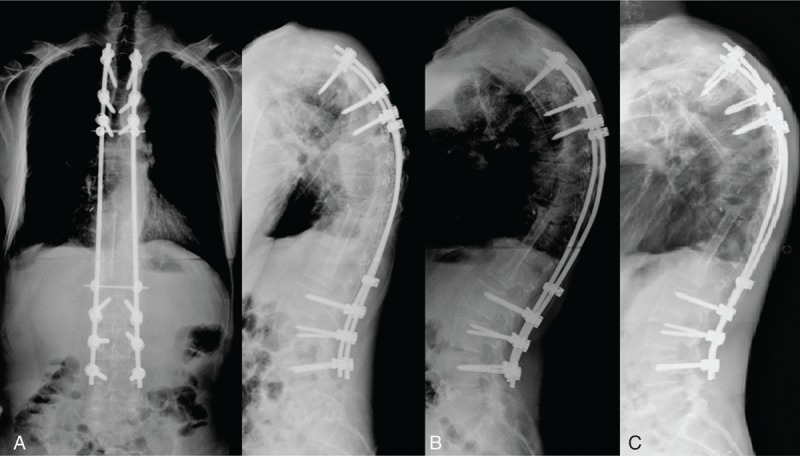
(A) Radiograph showing better alignment after single-stage combined anterior and posterior surgeries for complicated infectious spondylitis. (B) Bony incorporation between the implanted fibular allograft and host vertebral body was noted on the lateral radiograph 1 year later. (C) The radiograph 3 years later revealed good anterior interbody fusion and acceptable sagittal alignment without hardware breakage.
TABLE 1.
Patient Demographic Data and Clinical Outcomes
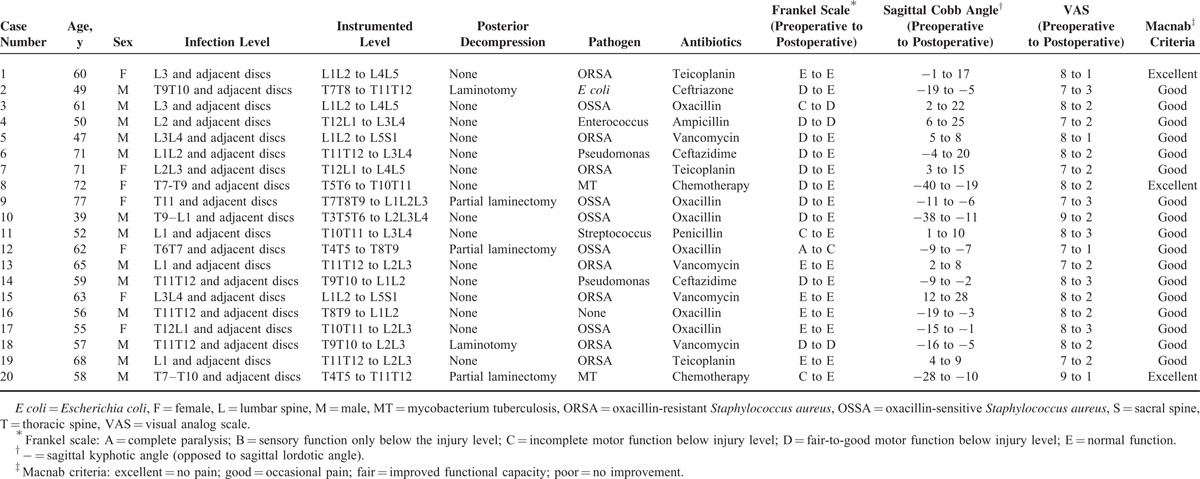
FIGURE 2.
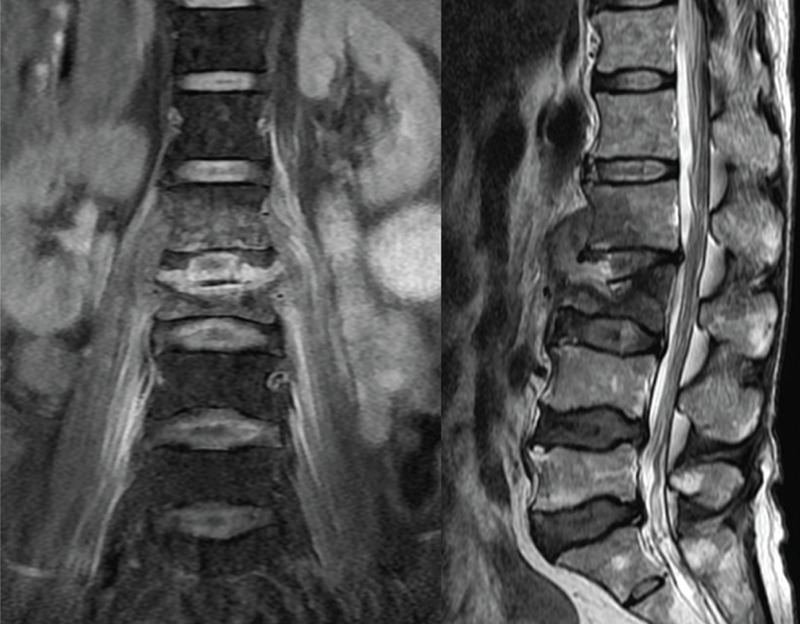
Coronal T2-weighted MRI revealed L3 infectious spondylitis with bilateral psoas muscle abscess extension. Sagittal MRI also showed L3 abscess extension beneath anterior longitudinal ligament. MRI = magnetic resonance imaging.
FIGURE 3.
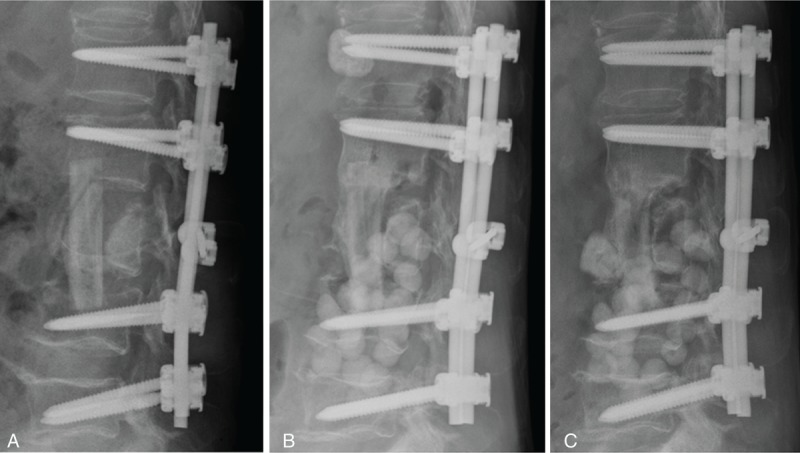
(A) Single-stage anterior extensive debridement and fibular allograft implantation followed by supplemental posterior instrumentation was performed to treat spinal infection and correct kyphotic deformity. (B) Anterior wound dehiscence occurred at 3 weeks later after initial surgery. Sensitive antibiotics cement beads based on the culture report was deposited in retroperitoneal space to avoid infection progression. (C) Bony incorporation between the implanted fibular allograft and host vertebral body was noted on the lateral radiograph 1 year later.
FIGURE 4.
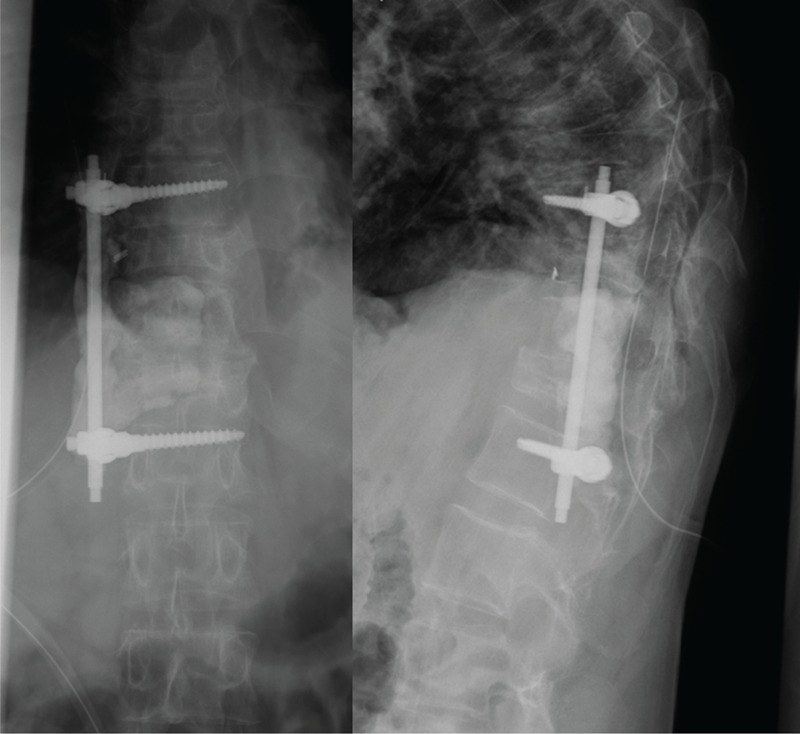
A 39-year-old man was transferred from other hospital because of sepsis after anterior reconstruction surgery for his ankylosing spondylitis. The lateral radiograph showed the bone cement and associated anterior instrumentation (Tri-Fix; A-Spine, Taipei, Taiwan) was used to restore his multilevel fractured vertebrae.
FIGURE 5.
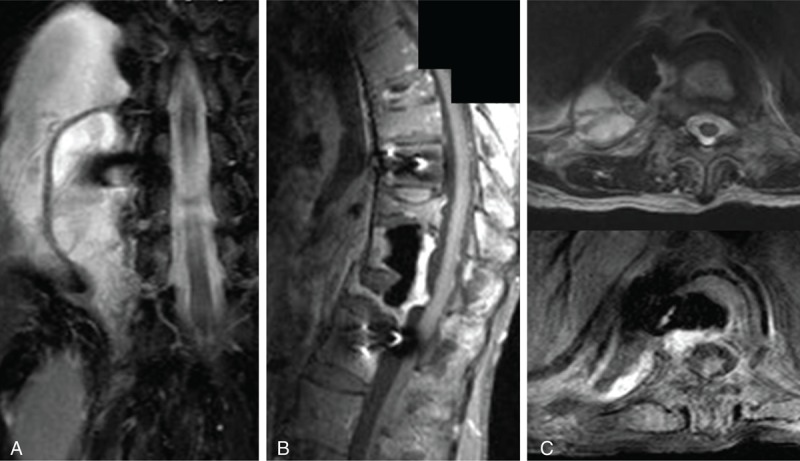
(A) Coronal T2-weighted MRI demonstrated a large amount of abscess surrounding the bone cement and Tri-Fix (A-Spine, Taipei, Taiwan) anterior instrument. (B) Sagittal T1-weighted MRI with gadolinium enhancement showed T11–T12 epidural abscess extension. (C) Axial MRI with or without gadolinium enhancement also verified an abundant abscess paravertebral accumulation. MRI = magnetic resonance imaging.
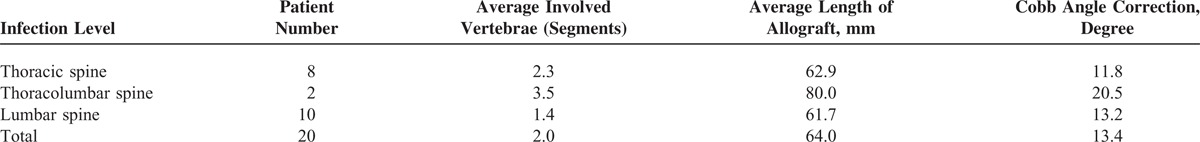
The average number of segments of resected vertebrae was 2.0 (2.3 in the thoracic vertebrae, 3.5 in the thoracolumbar, and 1.4 in the lumbar). The average length of the allograft for reconstruction was 64.0 mm (62.9 in the thoracic spine, 80.0 in the thoracolumbar, and 61.7 in the lumbar). Kyphotic deformity improved in all patients, with an average angle correction of 13.4° (11.8° in the thoracic spine, 20.5° in the thoracolumbar, and 13.2° in the lumbar) (Table 2). The average operation time was 4 hours and 26 minutes (from 3 hours 38 minutes to 7 hours 12 minutes). The average blood loss was 850 mL (from 350 to 2200 mL). The average hospital stay was 6.5 weeks (from 3 to 9 weeks).
TABLE 2.
Summary of Involved Vertebrae, Implanted Allograft Length, and Cobb Angle Correction
Causative bacteria were isolated in 16 (80%) of 20 computed tomography-guided biopsy cultures. Nine of the 16 patients were infected with Staphylococcus aureus, including 5 with the oxacillin-resistant strain and 4 with the oxacillin-sensitive strain. Two patients had Pseudomonas aeruginosa infection, 2 had Mycobacterium tuberculosis, and 3 had Streptococcus viridans, Enterococcus faecalis, and Escherichia coli infection, respectively. Intravenous antibiotic therapy was administered based on the identified pathologic organism and the specific microbial sensitivities and continued for a minimum of 6 weeks postoperatively, as recommended by the Infection Department of E-DA Hospital. Oral antibiotics were not routinely utilized upon completion of the 6 to 8-week course of intravenous antibiotics. Anti-tuberculous chemotherapy was administered at outpatient clinics for at least 12 months. Three of the 4 patients with negative findings finally had positive culture results by open biopsy, which matched the preoperative positive blood culture data, and then underwent sensitive antibiotics therapy. One patient with negative culture results received a full course of broad-spectrum antibiotics. The elevated CRP values returned to normal within a mean period of 4.7 weeks in these patients, and the elevated ESR irregularly decreased to half of the original pretreatment values within a mean period of 3.3 weeks (Figure 7).
FIGURE 7.
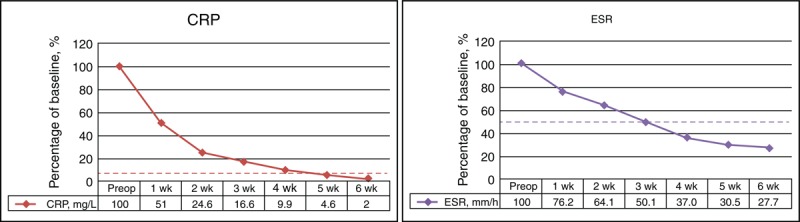
Percentage changes in serological values, before and after single-stage combined anterior and posterior surgeries in patients with complicated infectious spondylitis.
There was no implant breakage or allograft dislodgement. There was also no evidence of nonunion or pseudoarthrosis at junctions of implanted allograft and host bone. One patient experienced posterior superficial wound infection and 1 had anterior wound dehiscence. No recurrent infections were found among these patients during the postoperative follow-up (average, 51.2 months; range, from 38 to 75 months).
DISCUSSION
The mainstay procedure in treating patients with spinal infection is to make a diagnosis, isolate the offending pathogen, and prescribe effective antibiotics therapy. Nonsurgical treatment is usually adequate for infectious spondylodiscitis in those cases detected early. However, a delay in diagnosis and treatment is common in all forms of spinal infection because of the early indolent course.1–4 Rezai et al reported that 25% of patients initially treated nonsurgically had medical therapy failure.12 Przybylski and Sharan13 found that some patients with pyogenic discitis and vertebral osteomyelitis developed progressive biomechanical instability related pain, despite the provision of nonsurgical management. The enrolled patients in this study all had complicated infectious spondylitis that included at least 1-level vertebral osteomyelitis with pathological fracture and adjacent discitis. Significant vertebral body destruction resulted in spinal instability, progressive kyphotic deformity, intractable back pain, neurological deficit, and large epidural abscess accumulation. Single-stage combined anterior and posterior procedures were used to treat these patients, including an anterior approach for initially extensive debridement and subsequent tubular allograft implantation, and an additional posterior approach for supplemental pedicle screw instrumentation to maintain secure stability and physiologic alignment.
Anterior decompression alone with primary bone grafting, as described by Hodgson and Stock14 for the treatment of tuberculous spondylitis, was effective in most cases. In 1950s, this procedure was typically followed by bed rest, Stryker-frame confinement, or cast immobilization. In 1973, Kemp et al15 successfully treated 14 patients diagnosed with nontuberculous spondylitis with anterior debridement and bone grafting. Thereafter, several studies reported that this procedure was also effective in patients with pyogenic spondylitis.3,16 As a general rule, implantation of an instrument into an infected field should be avoided. However, instrumentation of some concomitantly unstable and infected tibia or femur fractures seems to facilitate control of the infection and promote fracture healing.9–11 In 1979, Fountain17 first described a single-stage debridement and fixation procedure for the treatment of lumbar pyogenic spondylitis. In addition to successful infection control, exploration during hardware removal at 12 months after surgery demonstrated that bony fusion had been attained. Klöckner and Valencia18 compared anterior debridement and bone grafting alone with a combined anterior and posterior procedure in terms of the physiological alignment of the segmental sagittal spinal profile. They concluded that anterior fusion alone seemed adequate for patients with single-level spondylodiscitis and only minor deformity, but additional posterior instrumentation was indicated for patients who had multiple-level spondylodiscitis, spinal deformity, or both.18
The average length of the allograft in this study was 64 mm, which restored an average 2 levels of vertebral body height in either the thoracic or the lumbar spine. During at least 3 years of follow-up, all bone grafts were incorporated with the adjacent vertebral body with different degrees of subsidence (average 2.5 mm) detected on radiographic examination, and there were no clinical symptoms. Various grafting techniques were used for vertebral body reconstruction, including fibular autograft, fibular allograft, and even antibiotics-impregnated polymethylmethacrylate.19–21 The latter was sometimes formed into antibiotics beads for use in the salvage of deep wound infection in our clinical practice. The use of vascularized bone graft seems to be a valuable reconstructive technique for the treatment of lower extremity osteomyelitis. Tu and Yen11 recommended that the indication for vascularized bone graft is a skeletal defect >6 cm in length. In a clinical series, Lee et al22 used vascularized fibula bone flaps to treat 6 patients with tumor or infection. Preoperative or postoperative spine radiation therapy was delivered to 4 of the 6 patients, and this along with fusion length was indicated for the use of vascularized fibula bone flaps. The authors concluded that this procedure provided an excellent adjunct in the treatment of difficult spinal arthrodesis, but did so with added potential morbidity and increased technical demand.22 The theory behind bone graft implantation following anterior vertebrectomy and debridement is to maintain or improve interspace height and to achieve bony healing, thus eliminating the pathological motion that can lead to instrument loosening or breakage. A corpectomy defect was reconstructed traditionally with a structural autologous bone graft from the iliac crest or fibula, which may cause an increase in permanent donor site morbidity up to 25%.23–26 The concern about donor site-related complications has led the authors to use allogenous bone grafts for the reconstruction of large bony defects. An ideal graft construct should have the ability of bony incorporation without collapse and with the fewest undesired complications. A cortical graft such as cadaveric humerus or fibula, which has a much higher modulus of elasticity and is less prone to collapse acutely over time, would be a good choice.27–29
Single-stage combined anterior and posterior approaches, as opposed to 2-stage surgeries, has resulted in less blood loss, a shorter anesthesia time, earlier mobilization, and less anxiety for the patient and family, as the patient undergoes only 1 operation. In this study, all 20 patients who received single-stage combined anterior and posterior surgeries for their complicated infectious spondylitis uneventfully recovered from the illness. All patients had effective back pain relief and significant neurologic function improvement. The average Cobb angle correction was 13.4° with good restoration of sagittal alignment. There were no complications related to the combined approach itself. One patient suffering from anterior wound dehiscence and infection postoperatively was successfully treated with anterior debridement and custom-made antibiotics bead deposition. Another patient with posterior superficial wound infection also responded to an additional procedure of wound debridement and repair. Przybylski and Sharan13 analyzed 10 clinical series involving 106 patients with spinal infection who received single-stage procedures. Deep wound infections were reported in 6.6% of patients, with most managed by debridement and secondary granulation. Superficial wound infections occurred in 2.8% of patients. A similar incidence of postoperative infection has been described in patients undergoing spinal instrumentation for spinal disorders other than infection treatment. Some studies even reported an incidence as high as 20% in instrumented spinal surgery.30,31 Therefore, a single-stage combined anterior and posterior surgery seems to be a reasonable procedure in consideration of complications of staged surgeries, if a cooperative surgical team and care unit are available.
There are some limitations in this study. First, only 20 cases were examined. Second, a retrospective study, such as this, does not include patients undergoing different treatment methods for comparison and lacks randomization. The feasibility and benefit of this single-stage combined anterior and posterior surgery using allograft reconstruction for complicated infectious spondylitis needs to be rigorously evaluated in a large patient population with prospectively controlled comparison groups. Third, most of the enrolled patients with complicated spinal infection had received different kinds of treatment, either conservative antibiotics or open surgery, and then transferred to our institute due to either previous failed treatment or progressive infection at their original hospital. After careful examination and evaluation, these eligible patients finally underwent a primary or revised surgery, with selective and therapeutic bias. However, either single-stage surgery or anterior reconstruction using allograft did not seem to predispose the patients to uncontrolled infection and difficulty in bony incorporation, based on the clinical results of this small patient population.
CONCLUSION
Complicated infectious spondylitis represents an infrequent infection with severe spinal destruction and is indicated for combined anterior spinal reconstruction and posterior fixation. Single-stage anterior debridement and allograft implantation followed by posterior pedicle screw instrumentation provide immediate stability, successful infection control, neurologic deficit recovery, more satisfactory alignment, and adequate back pain relief. Fibular allograft seems to be good alternative for anterior reconstruction, which can proceed to bony incorporation and avoid donor site morbidity. In addition to the biomechanical support of pedicle screw instrumentation and fibular bone grafting, an administration of at least 6 continuous weeks of pathogen-specific intravenous antibiotics is mandatory to eliminate the risk of postoperative infection recurrence and maintain good long-term outcomes.
Acknowledgment
The authors would like to express their warm thanks to all the staffs of E-DA Hospital who contributed to the study.
Footnotes
Abbreviations: CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, MRI = magnetic resonance imaging, VAS = visual analog scale.
T-CC wrote the original manuscript and contributed to the interpretation and presentation of the data. S-CY coordinated the study design, carried out the literature search, and was responsible for drafting of the manuscript. H-SC was responsible for the analysis and the interpretation of the data. Y-HK and Y-KT were responsible for the data acquisition. W-JC provided expert clinic advice in the field and critically revised the manuscript. All authors read and approved the final manuscript.
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.An HS, Seldomridge JA. Spinal infections: diagnostic tests and imaging studies. Clin Orthop Relat Res 2006; 444:27–33. [DOI] [PubMed] [Google Scholar]
- 2.Bonfiglio M, Lange TA, Kim YM. The Classic: pyogenic vertebral osteomyelitis: disk space infections. Clin Orthop Relat Res 2006; 444:4–8. [DOI] [PubMed] [Google Scholar]
- 3.Cahill DW, Love LC, Rechtine GR. Pyogenic osteomyelitis of the spine in the elderly. J Neurosurg 1991; 74:878–886. [DOI] [PubMed] [Google Scholar]
- 4.Carragee EJ. Pyogenic vertebral osteomyelitis. J Bone Joint Surg Am 1997; 79:874–880. [DOI] [PubMed] [Google Scholar]
- 5.Fraser RD, Osti OL, Vernon-Roberts B. Discitis after discography. J Bone Joint Surg Br 1987; 69:26–35. [DOI] [PubMed] [Google Scholar]
- 6.Maiuri F, Iaconetta G, Gallicchio B, et al. Spondylodiscitis. Clinical and magnetic resonance diagnosis. Spine 1997; 22:1741–1746. [DOI] [PubMed] [Google Scholar]
- 7.Perronne C, Saba J, Behloul Z, et al. Pyogenic and tuberculous spondylodiskitis (vertebral osteomyelitis) in 80 adult patients. Clin Infect Dis 1994; 19:746–750. [DOI] [PubMed] [Google Scholar]
- 8.Rohde V, Meyer B, Schaller C, et al. Spondylodiscitis after lumbar discectomy. Incidence and a proposal for prophylaxis. Spine 1998; 23:615–620. [DOI] [PubMed] [Google Scholar]
- 9.Jupiter JB, Bour CJ, May JW., Jr The reconstruction of defects in the femoral shaft with vascularized transfers of fibular bone. J Bone Joint Surg Am 1987; 69:365–374. [PubMed] [Google Scholar]
- 10.Lew DP, Waldvogel FA. Osteomyelitis. N Engl J Med 1997; 336:999–1007. [DOI] [PubMed] [Google Scholar]
- 11.Tu YK, Yen CY. Role of vascularized bone grafts in lower extremity osteomyelitis. Orthop Clin North Am 2007; 38:37–49. [DOI] [PubMed] [Google Scholar]
- 12.Rezai AR, Woo HH, Errico TJ, et al. Contemporary management of spinal osteomyelitis. Neurosurgery 1999; 44:1018–1025. [DOI] [PubMed] [Google Scholar]
- 13.Przybylski GJ, Sharan AD. Single-stage autogenous bone grafting and internal fixation in the surgical management of pyogenic discitis and vertebral osteomyelitis. J Neurosurg 2001; 94:1–7. [DOI] [PubMed] [Google Scholar]
- 14.Hodgson AR, Stock FE. Anterior spinal fusion a preliminary communication on the radical treatment of Pott's disease and Pott's paraplegia. Br J Surg 1956; 44:266–275. [DOI] [PubMed] [Google Scholar]
- 15.Kemp HB, Jackson JW, Jeremiah JD, et al. Anterior fusion of the spine for infective lesions in adults. J Bone Joint Surg Br 1973; 55:715–734. [PubMed] [Google Scholar]
- 16.Emery SE, Chan DP, Woodward HR. Treatment of hematogenous pyogenic vertebral osteomyelitis with anterior debridement and primary bone grafting. Spine 1989; 14:284–291. [PubMed] [Google Scholar]
- 17.Fountain SS. A single-stage combined surgical approach for vertebral resections. J Bone Joint Surg Am 1979; 61:1011–1017. [PubMed] [Google Scholar]
- 18.Klöckner C, Valencia R. Sagittal alignment after anterior debridement and fusion with or without additional posterior instrumentation in the treatment of pyogenic and tuberculous spondylodiscitis. Spine 2003; 28:1036–1042. [DOI] [PubMed] [Google Scholar]
- 19.Dietze DD, Jr, Fessler RG, Jacob RP. Primary reconstruction for spinal infections. J Neurosurg 1997; 86:981–989. [DOI] [PubMed] [Google Scholar]
- 20.Graziano GP, Sidhu KS. Salvage reconstruction in acute and late sequelae from pyogenic thoracolumbar infection. J Spinal Disord 1993; 6:199–207. [DOI] [PubMed] [Google Scholar]
- 21.Rath SA, Neff U, Schneider O, et al. Neurosurgical management of thoracic and lumbar vertebral osteomyelitis and discitis in adults: a review of 43 consecutive surgically treated patients. Neurosurgery 1996; 38:926–933. [DOI] [PubMed] [Google Scholar]
- 22.Lee MJ, Ondra SL, Mindea SA, et al. Indications and rationale for use of vascularized fibula bone flaps in cervical spine arthrodeses. Plast Reconstr Surg 2005; 116:1–7. [DOI] [PubMed] [Google Scholar]
- 23.Kurz LT, Garfin SR, Booth RE., Jr Harvesting autogenous iliac bone grafts. A review of complications and techniques. Spine 1989; 14:1324–1331. [DOI] [PubMed] [Google Scholar]
- 24.Sawin PD, Traynelis VC, Menezes AH. A comparative analysis of fusion rates and donor-site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg 1998; 88:255–265. [DOI] [PubMed] [Google Scholar]
- 25.Schnee CL, Freese A, Weil RJ, et al. Analysis of harvest morbidity and radiographic outcome using autograft for anterior cervical fusion. Spine 1997; 22:2222–2227. [DOI] [PubMed] [Google Scholar]
- 26.Summers BN, Eisenstein SM. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg Br 1989; 71:677–680. [DOI] [PubMed] [Google Scholar]
- 27.Currey JD. The effect of porosity and mineral content on the Young's modulus of elasticity of compact bone. J Biomech 1988; 21:131–139. [DOI] [PubMed] [Google Scholar]
- 28.Martin GJ, Jr, Haid RW, Jr, MacMillan M, et al. Anterior cervical discectomy with freeze-dried fibula allograft. Overview of 317 cases and literature review. Spine 1999; 24:852–858. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro S, Connolly P, Donnaldson J, et al. Cadaveric fibula, locking plate, and allogeneic bone matrix for anterior cervical fusions after cervical discectomy for radiculopathy or myelopathy. J Neurosurg 2001; 95:43–50. [DOI] [PubMed] [Google Scholar]
- 30.Mok JM, Guillaume TJ, Talu U, et al. Clinical outcome of deep wound infection after instrumented posterior spinal fusion: a matched cohort analysis. Spine 2009; 34:578–583. [DOI] [PubMed] [Google Scholar]
- 31.Rechtine GR, Bono PL, Cahill D, et al. Postoperative wound infection after instrumentation of thoracic and lumbar fractures. J Orthop Trauma 2001; 15:566–569. [DOI] [PubMed] [Google Scholar]


